Abstract
Chronic opioid use changes brain chemistry in areas related to reward processes, memory, decision-making, and addiction. Both neurons and astrocytes are affected, ultimately leading to dependence. Passiflora incarnata L. (Passifloraceae) is the basis of frequently used herbals to manage anxiety and insomnia, with proven central nervous system depressant effects. Anti-addiction properties of P. incarnata have been reported. The aim of this study was to investigate the effect of a commercial extract of Passiflora incarnata (Sintocalmy®, Aché Laboratory) in the naloxone-induced jum** mice model of morphine withdrawal. In addition, glial fibrillary acidic protein (GFAP) and S100 calcium-binding protein B (S100B) levels were assessed in the frontal cortex and hippocampus, and DNA damage was verified on blood cells. In order to improve solubilization a Sintocalmy methanol extract (SME) was used. SME is mainly composed by flavonoids isovitexin and vitexin. The effects of SME 50, 100 and 200 mg/kg (i.p.) were evaluated in the naloxone-induced withdrawal syndrome in mice. SME 50 and SME 100 mg/kg decreased naloxone-induced jum** in morphine-dependent mice without reducing locomotor activity. No alterations were found in GFAP levels, however SME 50 mg/kg prevented the S100B increase in the frontal cortex and DNA damage. This study shows anti-addiction effects for a commercial standardized extract of P. incarnata and suggests the relevance of proper clinical assessment.
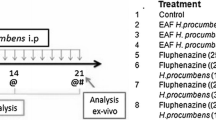
Graphic Abstract





Similar content being viewed by others
References
United Nations Office on Drugs and Crime (2019) World Drug Report 2019. United Nations publication
Bouzyk-Szutkiewicz J, Waszkiewicz N, Szulc A (2012) [Alcohol and psychiatric disorders]. Pol Merkur Lek Organ Pol Tow Lek 33:176–181
Rudd RA, Seth P, David F, Scholl L (2016) Increases in Drug and Opioid-Involved Overdose Deaths — United States, 2010–2015. MMWR Morb Mortal Wkly Rep 65:1445–1452. https://doi.org/10.15585/mmwr.mm655051e1
Seth P, Scholl L, Rudd RA, Bacon S (2018) Overdose Deaths Involving Opioids, Cocaine, and Psychostimulants — United States, 2015–2016. MMWR Morb Mortal Wkly Rep 67:349–358. https://doi.org/10.15585/mmwr.mm6712a1
Koob GF, Volkow ND (2016) Neurobiology of addiction: a neurocircuitry analysis. The lancet Psychiatry 3:760–773. https://doi.org/10.1016/S2215-0366(16)00104-8
Sulzer D (2011) How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron 69:628–649. https://doi.org/10.1016/j.neuron.2011.02.010
Volkow ND, Morales M (2015) The brain on drugs: from reward to addiction. Cell 162:712–725. https://doi.org/10.1016/J.CELL.2015.07.046
Li J-H, Lin L-F (1998) Genetic toxicology of abused drugs: a brief review. Mutagenesis 13:557–565. https://doi.org/10.1093/mutage/13.6.557
Tsujikawa H, Shoda T, Mizota T, Fukuda K (2009) Morphine induces DNA damage and P53 activation in CD3 + T cells. Biochim Biophys Acta - Gen Subj 1790:793–799. https://doi.org/10.1016/j.bbagen.2009.04.011
Feng Y-M, Jia Y-F, Su L-Y et al (2013) Decreased mitochondrial DNA copy number in the hippocampus and peripheral blood during opiate addiction is mediated by autophagy and can be salvaged by melatonin. Autophagy 9:1395–1406. https://doi.org/10.4161/auto.25468
Reynolds AR, Williams LA, Saunders MA, Prendergast MA (2015) Group 1 mGlu-family proteins promote neuroadaptation to ethanol and withdrawal-associated hippocampal damage. Drug Alcohol Depend 156:213–220. https://doi.org/10.1016/j.drugalcdep.2015.09.013
Perea G, Araque A (2005) Properties of Synaptically Evoked Astrocyte Calcium Signal Reveal Synaptic Information Processing by Astrocytes. J Neurosci 25:2192–2203. https://doi.org/10.1523/JNEUROSCI.3965-04.2005
Perea G, Sur M, Araque A (2014) Neuron-glia networks: integral gear of brain function. Front Cell Neurosci. https://doi.org/10.3389/fncel.2014.00378
Beitner-Johnson D, Guitart X, Nestler EJ (1993) Glial fibrillary acidic protein and the mesolimbic dopamine system: regulation by chronic morphine and Lewis-Fischer strain differences in the rat ventral tegmental area. J Neurochem 61:1766–1773
García-Sevilla JA, Ventayol P, Busquets X et al (1997) Marked decrease of immunolabelled 68 kDa neurofilament (NF-L) proteins in brains of opiate addicts. Neuroreport 8:1561–1565
Ferrer-Alcón M, García-Sevilla JA, Jaquet PE, et al (2000) Regulation of nonphosphorylated and phosphorylated forms of neurofilament proteins in the prefrontal cortex of human opioid addicts. J Neurosci Res 61:338–349. https://doi.org/10.1002/1097-4547(20000801)61:3<338::AID-JNR12>3.0.CO;2-5
Song P, Zhao ZQ (2001) The involvement of glial cells in the development of morphine tolerance. Neurosci Res 39:281–286
Marie-Claire C, Courtin C, Roques BP, Noble F (2004) Cytoskeletal genes regulation by chronic morphine treatment in rat striatum. Neuropsychopharmacology 29:2208–2215. https://doi.org/10.1038/sj.npp.1300513
Weber M, Scherf N, Kahl T et al (2013) Quantitative analysis of astrogliosis in drug-dependent humans. Brain Res 1500:72–87. https://doi.org/10.1016/j.brainres.2012.12.048
Bart G (2012) Maintenance medication for opiate addiction: the foundation of recovery. J Addict Dis 31:207–225. https://doi.org/10.1080/10550887.2012.694598
Blum K, Han D, Modestino EJ et al (2018) A systematic, intensive statistical investigation of data from the comprehensive analysis of reported drugs (CARD) for compliance and illicit opioid abstinence in substance addiction treatment with buprenorphine/naloxone. Subst Use Misuse 53:220–229. https://doi.org/10.1080/10826084.2017.1400064
Weiss RD, Rao V (2017) The Prescription opioid addiction treatment study: what have we learned. Drug Alcohol Depend 173 Suppl:S48–S54. https://doi.org/10.1016/j.drugalcdep.2016.12.001
Bisaga A, Popik P (2000) In search of a new pharmacological treatment for drug and alcohol addiction: N-methyl-D-aspartate (NMDA) antagonists. Drug Alcohol Depend 59:1–15
Leal MB, Michelin K, Souza DO, Elisabetsky E (2003) Ibogaine attenuation of morphine withdrawal in mice: role of glutamate N-methyl-d-aspartate receptors. Prog Neuro-Psychopharmacology Biol Psychiatry 27:781–785. https://doi.org/10.1016/S0278-5846(03)00109-X
Catania MA, Firenzuoli F, Crupi A et al (2003) Hypericum perforatum attenuates nicotine withdrawal signs in mice. Psychopharmacology 169:186–189. https://doi.org/10.1007/s00213-003-1492-0
Coskun I, Tayfun Uzbay I, Ozturk N, Ozturk Y (2006) Attenuation of ethanol withdrawal syndrome by extract of Hypericum perforatum in Wistar rats. Fundam Clin Pharmacol 20:481–488. https://doi.org/10.1111/j.1472-8206.2006.00432.x
Feily A, Abbasi N (2009) The inhibitory effect of Hypericum perforatum extract on morphine withdrawal syndrome in rat and comparison with clonidine. Phyther Res 23:1549–1552. https://doi.org/10.1002/ptr.2807
Khan M, Subhan F, Khan A et al (2014) Nature cures nature: Hypericum perforatum attenuates physical withdrawal signs in opium dependent rats. Pharm Biol 52:586–590. https://doi.org/10.3109/13880209.2013.854811
Groth-Marnat G, Leslie S, Renneker M (1996) Tobacco control in a traditional Fijian village: Indigenous methods of smoking cessation and relapse prevention. Soc Sci Med 43:473–477. https://doi.org/10.1016/0277-9536(95)00425-4
Seitz U, Schüle A, Gleitz J (1997) [3H]-Monoamine Uptake Inhibition Properties of Kava Pyrones. Planta Med 63:548–549. https://doi.org/10.1055/s-2006-957761
Steiner GG (2001) Kava as an anticraving agent: preliminary data. Pac Health Dialog 8:335–339
Capasso A, Sorrentino L (2005) Pharmacological studies on the sedative and hypnotic effect of Kava kava and Passiflora extracts combination. Phytomedicine 12:39–45. https://doi.org/10.1016/J.PHYMED.2004.03.006
Akhondzadeh S, Kashani L, Mobaseri M et al (2001) Passionflower in the treatment of opiates withdrawal: a double-blind randomized controlled trial. J Clin Pharm Ther 26:369–373
Dhawan K, Kumar S, Sharma A (2002) Reversal of morphine tolerance and dependence by Passiflora incarnata—a traditional medicine to combat morphine addiction. Pharm Biol 40:576–580. https://doi.org/10.1076/phbi.40.8.576.14660
Dhawan K, Kumar S, Sharma A (2002) Suppression of alcohol-cessation-oriented hyper-anxiety by the benzoflavone moiety of Passiflora incarnata Linneaus in mice. J Ethnopharmacol 81:239–244
Dhawan K, Dhawan S, Chhabra S (2003) Attenuation of benzodiazepine dependence in mice by a tri-substituted benzoflavone moiety of Passiflora incarnata Linneaus: a non-habit forming anxiolytic. J Pharm Pharm Sci A Publ Can Soc Pharm Sci Soc Can Des Sci Pharm 6:215–222
Dhawan K, Dhawan S, Sharma A (2004) Passiflora: a review update. J Ethnopharmacol 94:1–23. https://doi.org/10.1016/j.jep.2004.02.023
Neuwinger HD (2000) African traditional medicine: a dictionary of plant use and applications with supplement: search system for diseases. Medpharm Scientific Publishers, Stuttgart
Miroddi M, Calapai G, Navarra M et al (2013) Passiflora incarnata L.: Ethnopharmacology, clinical application, safety and evaluation of clinical trials. J Ethnopharmacol 150:791–804. https://doi.org/10.1016/j.jep.2013.09.047
Grundmann O, Wang J, McGregor G, Butterweck V (2008) Anxiolytic activity of a phytochemically characterized Passiflora incarnata extract is mediated via the GABAergic system. Planta Med 74:1769–1773. https://doi.org/10.1055/s-0028-1088322
Elsas S-M, Rossi DJ, Raber J et al (2010) Passiflora incarnata L. (Passionflower) extracts elicit GABA currents in hippocampal neurons in vitro, and show anxiogenic and anticonvulsant effects in vivo, varying with extraction method. Phytomedicine 17:940–949. https://doi.org/10.1016/j.phymed.2010.03.002
Appel K, Rose T, Fiebich B et al (2011) Modulation of the γ-aminobutyric acid (GABA) system by Passiflora incarnata L. Phyther Res 25:838–843. https://doi.org/10.1002/ptr.3352
Jawna-Zboińska K, Blecharz-Klin K, Joniec-Maciejak I et al (2016) Passiflora incarnata L. improves spatial memory, reduces stress, and affects neurotransmission in rats. Phyther Res PTR 30:781–789. https://doi.org/10.1002/ptr.5578
Schunck RVA, Macedo IC, Laste G et al (2017) Standardized Passiflora incarnata L. extract reverts the analgesia induced by alcohol withdrawal in rats. Phyther Res 31:1199–1208. https://doi.org/10.1002/ptr.5839
Vasudev V (1955) Dhanwantri Banoshdhi Visheshank. Gurukul Kangri Prakashak, Haridwar, India 364–366
Dhawan K, Kumar S, Sharma A (2001) Anti-anxiety studies on extracts of Passiflora incarnata Linneaus. J Ethnopharmacol 78:165–170. https://doi.org/10.1016/S0378-8741(01)00339-7
Maciel ÉS, Biasibetti R, Costa AP et al (2014) Subchronic oral administration of benzo[a]pyrene impairs motor and cognitive behavior and modulates S100B levels and MAPKs in rats. Neurochem Res 39:731–740. https://doi.org/10.1007/s11064-014-1261-y
Popik P, Layer RT, Fossom LH et al (1995) NMDA antagonist properties of the putative antiaddictive drug, ibogaine. J Pharmacol Exp Ther 275:753
Zarrindast M-R, Farzin D (1996) Nicotine attenuates naloxone-induced jum** behaviour in morphine-dependent mice. Eur J Pharmacol 298:1–6. https://doi.org/10.1016/0014-2999(95)00761-X
Paxinos G, Keith BJ, Franklin MA (2007) The Mouse Brain in Stereotaxic Coordinates, 3rd ed. Elsevier Science
Leite MC, Galland F, Brolese G et al (2008) A simple, sensitive and widely applicable ELISA for S100B: Methodological features of the measurement of this glial protein. J Neurosci Methods 169:93–99. https://doi.org/10.1016/j.jneumeth.2007.11.021
Tramontina F, Leite MC, Cereser K et al (2007) Immunoassay for glial fibrillary acidic protein: Antigen recognition is affected by its phosphorylation state. J Neurosci Methods 162:282–286. https://doi.org/10.1016/j.jneumeth.2007.01.001
Göethel G, Brucker N, Moro M A, et al (2014) Evaluation of genotoxicity in workers exposed to benzene and atmospheric pollutants. Mutat Res Toxicol Environ Mutagen 770:61–65. https://doi.org/10.1016/j.mrgentox.2014.05.008
Kerachian N, Alaee H, Gharavi-Naini M et al (2007) Effects of alcoholic extract of Avena sativa, Hypericum perforatum, Passiflora incarnata and Lavandula officinalis on symptoms of morphine withdrawal syndrome in rats. Physiol-Pharmacol 10:313–321
Zarrindast M-R, Mousa-Ahmadi E (1999) Effects of GABAergic system on naloxone-induced jum** in morphine-dependent mice. Eur J Pharmacol 381:129–133. https://doi.org/10.1016/S0014-2999(99)00546-4
Yuan L, Wang J, **ao HF et al (2012) Isoorientin induces apoptosis through mitochondrial dysfunction and inhibition of PI3K/Akt signaling pathway in HepG2 cancer cells. Toxicol Appl Pharmacol 265:83
Yuan L, Han X, Li W et al (2016) Isoorientin prevents hyperlipidemia and liver injury by regulating lipid metabolism, antioxidant capability, and inflammatory cytokine release in high-fructose-fed mice. J Agric Food Chem 64:2682–2689. https://doi.org/10.1021/acs.jafc.6b00290
Lam KY, Ling APK, Koh RY et al (2016) A review on medicinal properties of orientin. Adv Pharmacol Sci. https://doi.org/10.1155/2016/4104595
Koob GF, Maldonado R, Stinus L (1992) Neural substrates of opiate withdrawal. Trends Neurosci 15:186–191
Aman U, Subhan F, Shahid M et al (2016) Passiflora incarnata attenuation of neuropathic allodynia and vulvodynia apropos GABA-ergic and opioidergic antinociceptive and behavioural mechanisms. BMC Complement Altern Med. https://doi.org/10.1186/s12906-016-1048-6
Ayres ASFSJ, Santos WB, Junqueira-Ayres DD et al (2017) Monoaminergic neurotransmission is mediating the antidepressant-like effects of Passiflora edulis Sims fo. edulis. Neurosci Lett 660:79–85. https://doi.org/10.1016/j.neulet.2017.09.010
Donato R, Cannon BR, Sorci G et al (2013) Functions of S100 proteins. Curr Mol Med 13:24–57
Van Eldik LJ, Wainwright MS (2003) The Janus face of glial-derived S100B: beneficial and detrimental functions in the brain. Restor Neurol Neurosci 21:97–108
Brvar M, Ambrozic J, Osredkar J et al (2005) S100B protein in heroin overdose: a pilot study. Crit Care 9:P290. https://doi.org/10.1186/cc3353
Granstrem O, Adriani W, Shumilina M et al (2006) Specific changes in levels of autoantibodies to glutamate and opiate receptors induced by morphine administration in rats. Neurosci Lett 403:1–5. https://doi.org/10.1016/j.neulet.2006.04.017
Leite MC (2010) O envolvimento do cálcio e das junções gap na secreção de S100B em astrócitos de ratos. Universidade Federal do Rio Grande do Sul. Instituto de Ciências Básicas da Saúde. Programa de Pós-Graduação em Ciências Biológicas: Bioquímica
Girard M, Malauzat D, Nubukpo P (2019) Serum inflammatory molecules and markers of neuronal damage in alcohol-dependent subjects after withdrawal. World J Biol Psychiatry 20:76–90. https://doi.org/10.1080/15622975.2017.1349338
Wedekind D, Neumann K, Falkai P et al (2011) S100B and homocysteine in the acute alcohol withdrawal syndrome. Eur Arch Psychiatry Clin Neurosci 261:133–138. https://doi.org/10.1007/s00406-010-0121-2
Amaral GF, Dossa PD, Viebig LB et al (2016) Astrocytic expression of GFAP and serum levels of IL-1β and TNF-α in rats treated with different pain relievers. Brazilian J Pharm Sci 52:623–633. https://doi.org/10.1590/s1984-82502016000400006
Skrabalova J, Drastichova Z, Novotny J (2013) Morphine as a Potential Oxidative Stress-Causing Agent. Mini Rev Org Chem 10:367–372. https://doi.org/10.2174/1570193X113106660031
Barzilai A, Yamamoto K-I (2004) DNA damage responses to oxidative stress. DNA Repair 3:1109–1115. https://doi.org/10.1016/j.dnarep.2004.03.002
Xu B, Wang Z, Li G et al (2006) Heroin-Administered Mice Involved in Oxidative Stress and Exogenous Antioxidant-Alleviated Withdrawal Syndrome. Basic Clin Pharmacol Toxicol 99:153–161. https://doi.org/10.1111/j.1742-7843.2006.pto_461.x
Shafer DA, **e Y, Falek A (1994) Detection of opiate-enhanced increases in DNA damage, HPRT mutants, and the mutation frequency in human HUT-78 cells. Environ Mol Mutagen 23:37–44. https://doi.org/10.1002/em.2850230107
López-Alarcón C, Denicola A (2013) Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal Chim Acta 763:1–10. https://doi.org/10.1016/j.aca.2012.11.051
Kim JHH, Lee BC, Kim JHH et al (2005) The isolation and antioxidative effects of vitexin from Acer palmatum. Arch Pharm Res 28:195. https://doi.org/10.1007/BF02977715
Procházková D, Boušová I, Wilhelmová N (2011) Antioxidant and prooxidant properties of flavonoids. Fitoterapia 82:513–523
Fang An FA, **nxin Cao XC, Haiqi Qu HQ, Shuhua Wang SW (2015) Attenuation of oxidative stress of erythrocytes by the plant-derived flavonoids vitexin and apigenin. 724–732. https://doi.org/10.1691/ph.2015.5665
Lee EB, Kim JH, Cha Y-S et al (2015) Lifespan Extending and Stress Resistant Properties of Vitexin from Vigna angularis in Caenorhabditis elegans. Biomol Ther (Seoul) 23:582–589. https://doi.org/10.4062/biomolther.2015.128
Acknowledgements
The research was supported by the following Brazilian funding agencies: National Council for Scientific and Technological Development - CNPq; Committee for the Development of Higher Education Personnel—CAPES, Fundação de Amparo a Pesquisa do Rio Grande do Sul (FAPERGS- Processo 33416.465.34568.26032018), and PROPG/UFRGS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11064_2021_3237_MOESM1_ESM.tif
Figure S1. HPLC chromatogram of the Sintocalmy methanolic extract (SME). 4: isoschaftoside, 5: isoorientin, 6: orientin, 7: schaftoside, 10: saponarin, 11: vitexin and 12: isovitexin, previously published [44]. (TIF 198 kb)
Rights and permissions
About this article
Cite this article
dos Reis Izolan, L., da Silva, D.M., Oliveira, H.B.L. et al. Sintocalmy, a Passiflora incarnata Based Herbal, Attenuates Morphine Withdrawal in Mice. Neurochem Res 46, 1092–1100 (2021). https://doi.org/10.1007/s11064-021-03237-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03237-w