Abstract
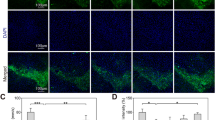
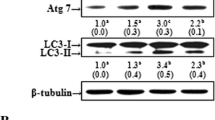
Dysfunction of autophagy, mitochondrial dynamics and endoplasmic reticulum (ER) stress are currently considered as major contributing factors in the pathogenesis of Parkinson’s disease (PD). Accumulation of oxidatively damaged cytoplasmic organelles and unfolded proteins in the lumen of the ER causes ER stress and it is associated with dopaminergic cell death in PD. Rotenone is a pesticide that selectively kills dopaminergic neurons by a variety of mechanism, has been implicated in PD. Geraniol (GE; 3,7-dimethylocta-trans-2,6-dien-1-ol) is an acyclic monoterpene alcohol occurring in the essential oils of several aromatic plants. In this study, we investigated the protective effect of GE on rotenone-induced mitochondrial dysfunction dependent oxidative stress leads to cell death in SK-N-SH cells. In addition, we assessed the involvement of GE on rotenone-induced dysfunction in autophagy machinery via α-synuclein accumulation induced ER stress. We found that pre-treatment of GE enhanced cell viability, ameliorated intracellular redox, preserved mitochondrial membrane potential and improves the level of mitochondrial complex-1 in rotenone treated SK-N-SH cells. Furthermore, GE diminishes autophagy flux by reduced autophagy markers, and decreases ER stress by reducing α-synuclein expression in SK-N-SH cells. Our results demonstrate that GE possess its neuroprotective effect via reduced rotenone-induced oxidative stress by enhanced antioxidant status and maintain mitochondrial function. Furthermore, GE reduced ER stress and improved autophagy flux in the neuroblastomal SK-N-SH cells. The present study could suggest that GE a novel therapeutic avenue for clinical intervention in neurodegenerative diseases especially for PD.









Similar content being viewed by others
References
Balch WE, Morimoto RI, Dillin A, Kelly JW (2008) Adapting proteostasis for disease intervention. Science 319:916–919
**ong N, Jia M, Chen C, **ong J, Zhang Z, Huang J, Hou L, Yang H, Cao X, Liang Z, Sun S, Lin Z, Wang T (2011) Potential autophagy enhancers attenuate rotenone-induced toxicity in SH-SY5Y. Neuroscience 199:292–302
Dodson M, Darley-Usmar V, Zhang J (2013) Cellular metabolic and autophagic pathways: traffic control by redox signaling. Free Radic Biol Med 63:207–221
Lee J, Giordano S, Zhang J (2012) Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J 441:523–540
Zhang J (2013) Autophagy and mitophagy in cellular damage control. Redox Biol 1:19–23
Dadakhujaev S, Noh HS, Jung EJ, Cha JY, Baek SM, Ha JH, Kim DR (2010) Autophagy protects the rotenone-induced cell death in alpha-synuclein overexpressing SH-SY5Y cells. Neurosci Lett 472:47–52
Bhutia SK, Dash R, Das SK, Azab B, Su ZZ, Lee SG, Grant S, Yacoub A, Dent P, Curiel DT, Sarkar D, Fisher PB (2010) Mechanism of autophagy to apoptosis switch triggered in prostate cancer cells by antitumor cytokine melanoma differentiation-associated gene 7/interleukin-24. Cancer Res 70:3667–3676
Imai Y, Soda M, Inoue H, Hattori N, Mizuno Y, Takahashi R (2001) An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell 105:891–902
Oh SH, Lim SC (2009) Endoplasmic reticulum stress-mediated autophagy/apoptosis induced by capsaicin (8-methyl-N-vanillyl-6-nonenamide) and dihydrocapsaicin is regulated by the extent of c-Jun NH2-terminal kinase/extracellular signal-regulated kinase activation in WI38 lung epithelial fibroblast cells. J Pharmacol Exp Ther 329:112–122
Higgins GC, Beart PM, Shin YS, Chen MJ, Cheung NS, Nagley P (2010) Oxidative stress: emerging mitochondrial and cellular themes and variations in neuronal injury. J Alzheimers Dis 20(Suppl 2):S453–S473
Martinez TN, Greenamyre JT (2012) Toxin models of mitochondrial dysfunction in Parkinson’s disease. Antioxid Redox Signal 16:920–934
Licker V, Kovari E, Hochstrasser DF, Burkhard PR (2009) Proteomics in human Parkinson’s disease research. J Proteomics 73:10–29
Dexter DT, Carter CJ, Wells FR, Javoy-Agid F, Agid Y, Lees A, Jenner P, Marsden CD (1989) Basal lipid peroxidation in substantia nigra is increased in Parkinson’s disease. J Neurochem 52:381–389
Levites Y, Weinreb O, Maor G, Youdim MB, Mandel S (2001) Green tea polyphenol (-)-epigallocatechin-3-gallate prevents N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurodegeneration. J Neurochem 78:1073–1082
Moussaoui S, Obinu MC, Daniel N, Reibaud M, Blanchard V, Imperato A (2000) The antioxidant ebselen prevents neurotoxicity and clinical symptoms in a primate model of Parkinson’s disease. Exp Neurol 166:235–245
Pan T, Rawal P, Wu Y, **e W, Jankovic J, Le W (2009) Rapamycin protects against rotenone-induced apoptosis through autophagy induction. Neuroscience 164:541–551
Wu Y, Li X, Zhu JX, **e W, Le W, Fan Z, Jankovic J, Pan T (2011) Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson’s disease. Neurosignals 19:163–174
Fukui M, Choi HJ, Zhu BT (2010) Mechanism for the protective effect of resveratrol against oxidative stress-induced neuronal death. Free Radic Biol Med 49:800–813
Bastos JF, Moreira IJ, Ribeiro TP, Medeiros IA, Antoniolli AR, De Sousa DP, Santos MR (2010) Hypotensive and vasorelaxant effects of citronellol, a monoterpene alcohol, in rats. Basic Clin Pharmacol Toxicol 106:331–337
Guimaraes AG, Quintans JS, Quintans LJ Jr (2013) Monoterpenes with analgesic activity–a systematic review. Phytother Res 27:1–15
Menezes IA, Barreto CM, Antoniolli AR, Santos MR, de Sousa DP (2010) Hypotensive activity of terpenes found in essential oils. Z Naturforsch C 65:562–566
Tiwari M, Kakkar P (2009) Plant derived antioxidants: geraniol and camphene protect rat alveolar macrophages against t-BHP induced oxidative stress. Toxicol In vitro 23:295–301
Prasad SN, Muralidhara (2014) Neuroprotective effect of geraniol and curcumin in an acrylamide model of neurotoxicity in Drosophila melanogaster: relevance to neuropathy. J Insect Physiol 60:7–16
Sadraei H, Asghari G, Emami S (2013) Inhibitory effect of Rosa damascena Mill flower essential oil, geraniol and citronellol on rat ileum contraction. Res Pharm Sci 8:17–23
Rekha KR, Selvakumar GP (2014) Gene expression regulation of Bcl2, Bax and cytochrome-C by geraniol on chronic MPTP/probenecid induced C57BL/6 mice model of Parkinson’s disease. Chem Biol Interact 217:57–66
Rekha KR, Selvakumar GP, Santha K, Inmozhi Sivakamasundari R (2013) Geraniol attenuates alpha-synuclein expression and neuromuscular impairment through increase dopamine content in MPTP intoxicated mice by dose dependent manner. Biochem Biophys Res Commun 440:664–670
Rekha KR, Selvakumar GP, Sethupathy S, Santha K, Sivakamasundari RI (2013) Geraniol ameliorates the motor behavior and neurotrophic factors inadequacy in MPTP-induced mice model of Parkinson’s disease. J Mol Neurosci 51:851–862
Kavitha M, Manivasagam T, Essa MM, Tamilselvam K, Selvakumar GP, Karthikeyan S, Thenmozhi JA, Subash S (2014) Mangiferin antagonizes rotenone: induced apoptosis through attenuating mitochondrial dysfunction and oxidative stress in SK-N-SH neuroblastoma cells. Neurochem Res 39:668–676
Tamilselvam K, Braidy N, Manivasagam T, Essa MM, Prasad NR, Karthikeyan S, Thenmozhi AJ, Selvaraju S, Guillemin GJ (2013) Neuroprotective effects of hesperidin, a plant flavanone, on rotenone-induced oxidative stress and apoptosis in a cellular model for Parkinson’s disease. Oxid Med Cell Longev. https://doi.org/10.1155/2013/102741
Hafer K, Konishi T, Schiestl RH (2008) Radiation-induced long-lived extracellular radicals do not contribute to measurement of intracellular reactive oxygen species using the dichlorofluorescein method. Radiat Res 169:469–473
Yu S, Liu M, Gu X, Ding F (2008) Neuroprotective effects of salidroside in the PC12 cell model exposed to hypoglycemia and serum limitation. Cell Mol Neurobiol 28:1067–1078
Niehaus WG Jr, Samuelsson B (1968) Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem 6:126–130
Del Maestro RF, Vaithilingam IS, McDonald W (1995) Degradation of collagen type IV by C6 astrocytoma cells. J Neuroncol 24:75–81
Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 21:130–132
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Vander Heiden MG, Chandel NS, Williamson EK, Schumacker PT, Thompson CB (1997) Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell 91:627–637
Hovius R, Lambrechts H, Nicolay K, de Kruijff B (1990) Improved methods to isolate and subfractionate rat liver mitochondria. Lipid composition of the inner and outer membrane. Biochim Biophys Acta 1021:217–226
Darzynkiewicz Z, Li X, Gong J (1994) Assays of cell viability: discrimination of cells dying by apoptosis. Methods Cell Biol 41:15–38
Jayaraj RL, Tamilselvam K, Manivasagam T, Elangovan N (2013) Neuroprotective effect of CNB-001, a novel pyrazole derivative of curcumin on biochemical and apoptotic markers against rotenone-induced SK-N-SH cellular model of Parkinson’s disease. J Mol Neurosci 51:863–870
Kavitha M, Nataraj J, Essa MM, Memon MA, Manivasagam T (2013) Mangiferin attenuates MPTP induced dopaminergic neurodegeneration and improves motor impairment, redox balance and Bcl-2/Bax expression in experimental Parkinson’s disease mice. Chem Biol Interact 206:239–247
Fiskum G, Starkov A, Polster BM, Chinopoulos C (2003) Mitochondrial mechanisms of neural cell death and neuroprotective interventions in Parkinson’s disease. Ann NY Acad Sci 991:111–119
Song JX, Choi MY, Wong KC, Chung WW, Sze SC, Ng TB, Zhang KY (2012) Baicalein antagonizes rotenone-induced apoptosis in dopaminergic SH-SY5Y cells related to Parkinsonism. Chin Med 7:1
Selvakumar GP, Janakiraman U, Essa MM, Justin Thenmozhi A, Manivasagam T (2014) Escin attenuates behavioral impairments, oxidative stress and inflammation in a chronic MPTP/probenecid mouse model of Parkinson’s disease. Brain Res 1585:23–36
Moon HE, Paek SH (2015) Mitochondrial dysfunction in Parkinson’s disease. Exp Neurobiol 24:103–116
Szewczyk A, Wojtczak L (2002) Mitochondria as a pharmacological target. Pharmacol Rev 54:101–127
Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT (2003) Mechanism of toxicity in rotenone models of Parkinson’s disease. J Neurosci 23:10756–10764
Ezoulin MJ, Ombetta JE, Dutertre-Catella H, Warnet JM, Massicot F (2008) Antioxidative properties of galantamine on neuronal damage induced by hydrogen peroxide in SK-N-SH cells. Neurotoxicology 29:270–277
Zhao HW, Li XY (2002) Ginkgolide A, B, and huperzine A inhibit nitric oxide-induced neurotoxicity. Int Immunopharmacol 2:1551–1556
Sian J, Dexter DT, Lees AJ, Daniel S, Jenner P, Marsden CD (1994) Glutathione-related enzymes in brain in Parkinson’s disease. Ann Neurol 36:356–361
Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, Jenner P, Marsden CD (1994) Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol 36:348–355
Liu J, Ames BN (2005) Reducing mitochondrial decay with mitochondrial nutrients to delay and treat cognitive dysfunction, Alzheimer’s disease, and Parkinson’s disease. Nutr Neurosci 8:67–89
Liu W, Vives-Bauza C, Acin-Perez R, Yamamoto A, Tan Y, Li Y, Magrane J, Stavarache MA, Shaffer S, Chang S, Kaplitt MG, Huang XY, Beal MF, Manfredi G, Li C (2009) PINK1 defect causes mitochondrial dysfunction, proteasomal deficit and alpha-synuclein aggregation in cell culture models of Parkinson’s disease. PLoS ONE 4:e4597
Betarbet R, Sherer TB, Di Monte DA, Greenamyre JT (2002) Mechanistic approaches to Parkinson’s disease pathogenesis. Brain Pathol 12:499–510
Shangguan WJ, Zhang YH, Li ZC, Tang LM, Shao J, Li H (2017) Naringin inhibits vascular endothelial cell apoptosis via endoplasmic reticulum stress and mitochondrialmediated pathways and promotes intraosseous angiogenesis in ovariectomized rats. Int J Mol Med 40:1741–1749
Watabe M, Nakaki T (2004) Rotenone induces apoptosis via activation of bad in human dopaminergic SH-SY5Y cells. J Pharmacol Exp Ther 311:948–953
Kim HJ, Song JY, Park HJ, Park HK, Yun DH, Chung JH (2009) Naringin protects against rotenone-induced apoptosis in human neuroblastoma SH-SY5Y cells. Korean J Physiol Pharmacol 13:281–285
Chu CT, Ji J, Dagda RK, Jiang JF, Tyurina YY, Kapralov AA, Tyurin VA, Yanamala N, Shrivastava IH, Mohammadyani D, Wang KZQ, Zhu J, Klein-Seetharaman J, Balasubramanian K, Amoscato AA, Borisenko G, Huang Z, Gusdon AM, Cheikhi A, Steer EK, Wang R, Baty C, Watkins S, Bahar I, Bayir H, Kagan VE (2013) Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat Cell Biol 15:1197–1205
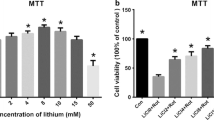
Hou L, **ong N, Liu L, Huang J, Han C, Zhang G, Li J, Xu X, Lin Z, Wang T (2015) Lithium protects dopaminergic cells from rotenone toxicity via autophagy enhancement. BMC Neurosci 16:82
Moors TE, Hoozemans JJ, Ingrassia A, Beccari T, Parnetti L, Chartier-Harlin MC, van de Berg WD (2017) Therapeutic potential of autophagy-enhancing agents in Parkinson’s disease. Mol Neurodegener 12:11
Mizushima N (2007) Autophagy: process and function. Genes Dev 21:2861–2873
Yorimitsu T, Nair U, Yang Z, Klionsky DJ (2006) Endoplasmic reticulum stress triggers autophagy. J Biol Chem 281:30299–30304
Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC (2007) Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J Biol Chem 282:5641–5652
Sarkar S, Perlstein EO, Imarisio S, Pineau S, Cordenier A, Maglathlin RL, Webster JA, Lewis TA, O’Kane CJ, Schreiber SL, Rubinsztein DC (2007) Small molecules enhance autophagy and reduce toxicity in Huntington’s disease models. Nat Chem Biol 3:331–338
Cherra SJ III, Chu CT (2008) Autophagy in neuroprotection and neurodegeneration: a question of balance. Future Neurol 3:309–323
Yue Z, Wang QJ, Komatsu M (2008) Neuronal autophagy: going the distance to the axon. Autophagy 4:94–96
Meijer AJ, Codogno P (2006) Signalling and autophagy regulation in health, aging and disease. Mol Aspects Med 27:411–425
Levine B, Yuan J (2005) Autophagy in cell death: an innocent convict? J Clin Invest 115:2679–2688
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19:5720–5728
Bjorkoy G, Lamark T, Johansen T (2006) p62/SQSTM1: a missing link between protein aggregates and the autophagy machinery. Autophagy 2:138–139
Berrocal R, Vasudevaraju P, Indi SS, Sambasiva Rao KR, Rao KS (2014) In vitro evidence that an aqueous extract of Centella asiatica modulates alpha-synuclein aggregation dynamics. J Alzheimers Dis 39:457–465
McMurray CT (2001) Huntington’s disease: new hope for therapeutics. Trends Neurosci 24:S32–S38
Back SH, Kaufman RJ (2012) Endoplasmic reticulum stress and type 2 diabetes. Annu Rev Biochem 81:767–793
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8:519–529
Velculescu VE, Zhang L, Vogelstein B, Kinzler KW (1995) Serial analysis of gene expression. Science 270:484–487
Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334:1081–1086
Jiang M, Liu L, He X, Wang H, Lin W, Wang H, Yoon SO, Wood TL, Lu QR (2016) Regulation of PERK-eIF2alpha signalling by tuberous sclerosis complex-1 controls homoeostasis and survival of myelinating oligodendrocytes. Nat Commun 7:12185
Harding HP, Zhang Y, Ron D (1999) Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397:271–274
Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D (2000) Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 6:1099–1108
Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, Mori K (2000) ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol 20:6755–6767
Mori K (2003) Frame switch splicing and regulated intramembrane proteolysis: key words to understand the unfolded protein response. Traffic 4:519–528
Vilatoba M, Eckstein C, Bilbao G, Smyth CA, Jenkins S, Thompson JA, Eckhoff DE, Contreras JL (2005) Sodium 4-phenylbutyrate protects against liver ischemia reperfusion injury by inhibition of endoplasmic reticulum-stress mediated apoptosis. Surgery 138:342–351
Wang B, Heath-Engel H, Zhang D, Nguyen N, Thomas DY, Hanrahan JW, Shore GC (2008) BAP31 interacts with Sec61 translocons and promotes retrotranslocation of CFTRDeltaF508 via the derlin-1 complex. Cell 133:1080–1092
Chen LH, Jiang CC, Watts R, Thorne RF, Kiejda KA, Zhang XD, Hersey P (2008) Inhibition of endoplasmic reticulum stress-induced apoptosis of melanoma cells by the ARC protein. Cancer Res 68:834–842
Han BH, Holtzman DM (2000) BDNF protects the neonatal brain from hypoxic-ischemic injury in vivo via the ERK pathway. J Neurosci 20:5775–5781
Han G, Casson RJ, Chidlow G, Wood JP (2014) The mitochondrial complex I inhibitor rotenone induces endoplasmic reticulum stress and activation of GSK-3beta in cultured rat retinal cells. Invest Ophthalmol Vis Sci 55:5616–5628
Han X, Zhang P, Jiang R, **a F, Li M, Guo FJ (2014) Explore on the effect of ATF6 on cell growth and apoptosis in cartilage development. Histochem Cell Biol 142:497–509
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Hereby, the authors declare that they have no conflict of interest that might have influenced the views expressed in this manuscript.
Rights and permissions
About this article
Cite this article
Rekha, K.R., Inmozhi Sivakamasundari, R. Geraniol Protects Against the Protein and Oxidative Stress Induced by Rotenone in an In Vitro Model of Parkinson’s Disease. Neurochem Res 43, 1947–1962 (2018). https://doi.org/10.1007/s11064-018-2617-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-018-2617-5