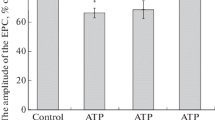
This report presents our studies of the ATP-dependent and calcium mechanisms of the effects of salicylates on the electrical activity of neurons in the mollusk Helix albescens Rossm. Adenosine triphosphate (5·10–4 M) added simultaneously with salicylates in the extracellular medium significantly modified their neurotropic effects, eliminating the nonselective suppression of neuron spike activity by acetylsalicylic acid and increasing the activatory influences of cobalt and zinc acetylsalicylates. Blockade of uptake of Ca2+ into the neuroplasm from the extracellular medium and intracellular depots with 5·10–5 M and 5·10–4 M CdCl2 and BaCl2 had no significant effect on neuron responses to salicylates, suggesting that calcium mechanisms are not involved.
Similar content being viewed by others
References
A. A. Boldyrev, “The role of the Na+-Ca2+ pump in excitable tissues,” Zh. Sib. Feder. Univ. Biol., 3, No. 1, 206–225 (2008).
A. I. Vislobokov, Yu. D. Ignatov, and K. N. Mel’nikov, Pharmacological Modulation of Neuron Membrane Ion Channels, St. Petersburg State Medical University Press, St. Petersburg (2006).
T. Kh. Gainutdinova, D. I. Silant’eva, V. V. Andrianov, et al., “Effects of increases and decreases in extracellular calcium levels on the electrical properties of command neurons in trained snails,” Uchen. Zap. Kazan. Univ. Ser. Estestv. Nauki, 15, No. 2, 29–40 (2010).
M. M. Dale and J. K. Foreman, Textbook of Immunopharmacology [Russian translation], Meditsina, Moscow (1998).
A. A. Zamotailov and I. I. Korenyuk, Auth. Certif. No. 1164229 Ukraine, “A computer program for the recording, processing, and automated analysis of bioelectrical signals,” publ. Nov. 29, 2004, Byull., No. 11.
A. L. Zefirov and G. F. Sitdikova, “The ion channels of nerve endings,” Usp. Fiziol. Nauk., 33, No. 4, 3–33 (2002).
A. U. Ziganshin, “The role of ATP receptors (P2 receptors) in the nervous system,” Nevrol. Vestn. Bekht., 37, No. 1–2, 45–53 (2005).
N. I. Kononenko and IO. N. Osipenko, “Effects of cadmium ions on the volley electrical activity of an identified common snail neuron,” Neirofiziologiya, 15, No. 3, 2047–2054 (1983).
I. I. Korenyuk, D. R. Khusainov, and V. F. Shul’gin, “Effects of salicylic acid and its salts on electrical activity in common snail neurons,” Neirofiziologiya, 37, No. 2, 142–150 (2005).
I. I. Korenyuk, D. R. Khusainov, and V. F. Shul’gin, “Cobalt and zinc salicylates as functional analogs of initiating factor in the mollusk nervous system,” Neirofiziologiya, 38, No. 1, 11–18 (2006).
K. N. Mel’nikov, “Diversity and properties of calcium channels in excitable membranes,” Psikhofarmakol. Biol. Narkol., 6, No. 1–2, 1139–1155 (2006).
A. A. Naumova, L. S. Kurilova, and K. Voitsekhovich, “The effects of aspirin on Ca2+ signals induced by glutoxim in peritoneal macrophages,” Fundam. Nauka Klin. Med., 15, 389–390 (2012).
N. B. Pestov, R. I. Dmitriev, and M. I. Shakhporonov, “Regulation of plasma membrane Ca-ATPase,” Usp. Biol. Khim., 43, No. 1, 99–138 (2003).
A. M. Safullina, A. M. Kas’yanov, E. M. Sokolova, et al., “The modulatory effect of adenosine triphosphate on the oscillatory properties of hippocampal neurons in early ontogeny,” Zh. Vyssh. Nerv. Deyat., 53, No. 4, 446–450 (2003).
Yu. M. Usachev and S. L. Mironov, “Effects of strontium and barium ions on the calcium binding and transport system in nerve cells,” Neirofiziologiya, 21, No. 6, 820–826 (1989).
I. V. Cheretaev, I. I. Korenyuk, D. R. Khusainov, et al., “Effects of cobalt and zinc acetylsalicylates on the electrical activity of neurons,” Akt. Vopr. Biol. Med., 10, 106–109 (2012).
T. V. Yakovchuk, O. V. Katyushina, D. R. Khusainov, et al., “Antiinflammatory activity of salts of salicylic and acetylsalicylic acids,” Uch. Zap. Tavrich. Nats. Univ. im. Vernadskogo. Ser. Biol. Khim., 24, No. 2, 332–338 (2011).
M. G. Berridge, “Neuronal calcium signaling,” Neuron, 21, No. 1, 13–18 (1998).
M. Brini and E. Carafoli, “Calcium pumps in health and disease,” Physiol. Rev., 89, No. 4, 1341–1378 (2009).
G. Burnstock, “ATP and its metabolites as potent extracellular agents,” Curr. Top. Membr., 54, No. 1, 1–27 (2003).
G. Burnstock, “Physiology and pathophysiology of purinergic neurotransmission,” Physiol. Rev., 87, No. 2, 659–797 (2007).
R. Dipolo and L. Beauge, “Sodium-calcium exchanger: influence of metabolic regulation on ion carrier interactions,” Physiol. Rev., 86, No. 1, 155–203 (2006).
W. Graffrath, T. Kirschtein, H. Nawrath, and R. D. Treede, “Acetylsalicylic acid reduces heat responses in rat nociceptive primary sensory neurons – evidence for a new mechanism of action,” Neurosci. Lett., 320, No. 1–2, 61–64 (2002).
J. G. Hardman, L. E. Limbird, and A. G. Gilman, Goodman and Gilman’s The Pharmacological Basis of Therapeutics, McGraw-Hill, New York (2001).
M. C. Jeziorski, R. M. Greenberg, and P. A. Anderson, “The molecular biology of invertebrate voltage-gated Ca2+-channels,” J. Exp. Biol., 203, No. 5, 841–856 (2000).
K. S. Kits and H. D. Mansvelder, “Voltage gated calcium channels in molluscs: classification, Ca2+ dependent inactivation, modulation and functional roles,” Invert. Neurosci., 2, No. 1, 9–34 (1996).
N. A. Lozovaya, C. A. Vulfius, V. I. Ilyin, and I. V. Krasts, “Intracellular ATP modifies the voltage dependence of the fast transient outward K+ current in Lymnaea stagnalis neurons,” J. Physiol., 464, 441–445 (1993).
L. L. Moroz, J. R. Edwards, and S. Puthanveettil, “Neuronal transcriptome of Aplysia: neuronal compartments and circuitry,” Cell, 127, No. 7, 1453–1467 (2006).
I. Ott, B. Kircher, C. Bagowski, et al., “Modulation of the biological properties of aspirin by formation of a bioorganometallic derivative,” Angew. Chem. Int. Ed., 48, No. 6, 1160–1163 (2009).
A. B. Parekh and J. W. Putney, “Store-operated calcium channels,” Physiol. Rev., 85, No. 2, 757–810 (2005).
E. Perez-Reyes, “Molecular physiology of low-voltage-activated T-type calcium channels,” Physiol. Rev., 83, No. 1, 117–161 (2003).
J. Sokolik, I. Tumova, M. Blahova, et al., “Anti-inflammatory activities of copper (II) and zinc (II) 3. 6-dimethylsalicylates and their equimolar mixture,” Acta Facult. Farm. Univ. Comenianae, 53, 224–228 (2006).
Y. Y. Su, B. Luo, H. T. Wang, and L. Chen, “Differential effects of sodium salicylate on current-evoked firing of pyramidal neurons and fast-spiking interneurons in slices of rat auditory cortex,” Hear. Res., 253, No. 1–3, 60–66 (2009).
M.-P. Wu, L.-S. Kao, H.-T. Liao, and C.-Y. Pan, “Reverse mode Na+-Ca2+ exchangers trigger the release of Ca2+ from intracellular Ca2+ stores in cultured rat embryonic cortical neurons,” Brain Res., 128, No. 1, 41–51 (2008).
X. Zhang, P. Yang, Y. Cao, and Y. Sato, “Salicylate induced neural changes in the primary auditory cortex of awake cats,” Neuroscience, 172, No. 1, 232–245 (2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 101, No. 3, pp. 326–336, March, 2015.
Rights and permissions
About this article
Cite this article
Cheretaev, I.V., Korenyuk, I.I., Khusainov, D.R. et al. ATP-Dependent and Calcium Mechanisms of the Effects of Salicylates on Electrical Potentials in Neurons in the Mollusk Helix Albescens . Neurosci Behav Physi 46, 644–651 (2016). https://doi.org/10.1007/s11055-016-0291-0
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-016-0291-0