Abstract
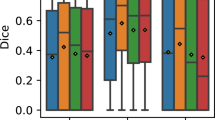
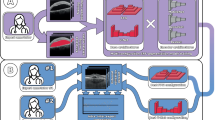
Age-related macular degeneration (AMD) is a degenerative retina condition that causes notable visual impairment in the central area of the visual field during its advanced stages. Manual segmentation of the retina layers and fluids in Optical coherence tomography (OCT) images is a dominant clinical practice but is time-consuming and labor-intensive due to the complexity of the images. Automated segmentation methods are necessary to facilitate efficient and quantitative assessment of the retina. However, the imbalanced distribution of classes or structures within OCT images, especially for smaller targets like intra-retinal fluids or specific layers, poses a challenge for automated segmentation methods. Algorithms may have difficulty accurately identifying and segmenting these smaller structures. The primary objective of this research is to develop segmentation models that can adapt to different pathologies, and stages of AMD disease progression while maintaining generalizability. The aim is to overcome challenges related to class imbalance, noise artifacts, intensity variations, and the scarcity of annotated OCT scans while ensuring precise and efficient segmentation. To achieve our aim, we propose a new architecture inspired by U-Net + + architecture and a Spatially adaptive denormalization Unit with a class-guided module. The segmentation is performed in two stages, to segment the layers and fluids. The proposed architecture results demonstrate significant improvement in AMD segmentation. It effectively handles class imbalance, leading to more accurate and reliable segmentation results, especially in the imbalanced classes with a Dice score of Pigment Epithelial Detachment (PED), Subretinal Fluid (SRF), Intraretinal Fluid (IRF) were 0.897, 0.927, and 0.787, respectively. Comparative analysis with other cutting-edge architectures highlights the strengths of our approach in addressing the challenges of automatic segmentation in medical images.
















Similar content being viewed by others
Data availability
The data used in this paper is available on request to corresponding author.
References
Zetterberg M (2016) Age-related eye disease and gender. Maturitas 83:19–26
Pascolini D, Mariotti SP (May 2012) Global estimates of visual impairment: 2010. Br J Ophthalmol 96(5):614–618. https://doi.org/10.1136/bjophthalmol-2011-300539
Jager RD, Mieler WF, Miller JW (2008) Age-related macular degeneration. N Engl J Med 358(24):2606–2617
Wong WL et al (2014) Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2:e106–e116
Wintergerst MWM et al (Jul. 2017) Algorithms for the Automated Analysis of Age-Related Macular Degeneration Biomarkers on Optical Coherence Tomography: a systematic review. Translational Vis Sci Technol 6(4):10. https://doi.org/10.1167/tvst.6.4.10
Chen Z, Mo Y, Ouyang P, Shen H, Li D, Zhao R (2019) Retinal vessel optical coherence tomography images for anemia screening. Med Biol Eng Comput 57(4):953–966. https://doi.org/10.1007/s11517-018-1927-8
Zheng M, Zhi K, Zeng J, Tian C, You L (2022) A Hybrid CNN for Image Denoising. J Artif Intell 2(3):3. https://doi.org/10.37965/jait.2022.0101
Chakraborty R, Verma G, Namasudra S (Jul. 2021) IFODPSO-based multi-level image segmentation scheme aided with Masi Entropy. J Ambient Intell Hum Comput 12(7):7793–7811. https://doi.org/10.1007/s12652-020-02506-w
Bhatia M, Bhatia S, Hooda M, Namasudra S, Taniar D (2022) Analyzing and classifying MRI images using robust mathematical modeling. Multimed Tools Appl 81(26):37519–37540. https://doi.org/10.1007/s11042-022-13505-8
Laghmati S, Hamida S, Hicham K, Cherradi B, Tmiri A (2023) An improved breast cancer disease prediction system using ML and PCA. Multimed Tools Appl pp. 1–37. https://doi.org/10.1007/s11042-023-16874-w
Das SK, Namasudra S, Kumar A, Moparthi NR (2023) AESPNet: Attention Enhanced Stacked Parallel Network to improve automatic Diabetic Foot Ulcer identification. Image Vision Comput 138(C). https://doi.org/10.1016/j.imavis.2023.104809
Singal G, Kushwaha R, Veeramsetty V, Badal T, Lamba S (2023) RoadWay. Multimed Tools Appl 82(4):4965–4978
Nawaz SA, Li J, Bhatti UA, Shoukat MU, Ahmad RM (2022) AI-based object detection latest trends in remote sensing, multimedia and agriculture applications. Front Plant Sci 13:1041514. https://doi.org/10.3389/fpls.2022.1041514
Srinivasan PP, Heflin SJ, Izatt JA, Arshavsky VY, Farsiu S (Feb. 2014) Automatic segmentation of up to ten layer boundaries in SD-OCT images of the mouse retina with and without missing layers due to pathology. Biomed Opt Express 5(2):348. https://doi.org/10.1364/BOE.5.000348
Chiu SJ, Allingham MJ, Mettu PS, Cousins SW, Izatt JA, Farsiu S (Apr. 2015) Kernel regression based segmentation of optical coherence tomography images with diabetic macular edema. Biomed Opt Express 6(4):1172. https://doi.org/10.1364/BOE.6.001172
Freeman SR (2010) Optical Coherence Tomography-Raster Scanning And Manual Segmentation In Determining Drusen Volume In Age-Related Macular Degeneration. Retina 30(3):431–435. https://doi.org/10.1097/IAE.0b013e3181bd2f94
Parra-Mora E, Cazañas-Gordón A, Proença R, da Silva Cruz LA (2021) Epiretinal Membrane Detection in Optical Coherence Tomography Retinal images using deep learning. IEEE Access 99201–99219. https://doi.org/10.1109/ACCESS.2021.3095655
Kugelman J et al (Oct. 2020) Retinal boundary segmentation in Stargardt Disease Optical Coherence Tomography images using Automated Deep Learning. Translational Vis Sci Technol 9(11):12. https://doi.org/10.1167/tvst.9.11.12
Cazañas-Gordón A, Mora EP, da Silva Cruz L (2021) Ensemble Learning Approach to Retinal Thickness Assessment in Optical Coherence Tomography. IEEE Access 9:67349–67363. https://doi.org/10.1109/ACCESS.2021.3076427
Hassan B et al (2021) Deep learning based joint segmentation and characterization of multi-class retinal fluid lesions on OCT scans for clinical use in anti-VEGF therapy. Comput Biol Med 136:104727. https://doi.org/10.1016/j.compbiomed.2021.104727
Daanouni O, Cherradi B, Tmiri A (2022) NSL-MHA-CNN: A novel CNN Architecture for Robust Diabetic Retinopathy Prediction against adversarial attacks’. IEEE Access 10:103987–103999. https://doi.org/10.1109/ACCESS.2022.3210179
Daanouni O, Cherradi B, Tmiri A (2021) Self-Attention Mechanism for Diabetic Retinopathy Detection. pp. 79–88. https://doi.org/10.1007/978-3-030-53440-0_10
Daanouni O, Cherradi B, Tmiri A (2021) Automatic Detection of Diabetic Retinopathy Using Custom CNN and Grad-CAM. In: Advances on Smart and Soft Computing, vol. 1188, F. Saeed, T. Al-Hadhrami, F. Mohammed, and E. Mohammed (eds), in Advances in Intelligent Systems and Computing, vol. 1188., Singapore: Springer Singapore, pp. 15–26. https://doi.org/10.1007/978-981-15-6048-4_2
Chen R, Pu D, Tong Y, Wu M (2022) Image-denoising algorithm based on improved K-singular value decomposition and atom optimization. CAAI Trans Intell Technol 7(1):117–127. https://doi.org/10.1049/cit2.12044
Melinščak M, Radmilović M, Vatavuk Z, Lončarić S (2021) Annotated retinal optical coherence tomography images (AROI) database for joint retinal layer and fluid segmentation. Automatika 62(3–4):375–385. https://doi.org/10.1080/00051144.2021.1973298
Wei H, Peng P (2020) The segmentation of Retinal Layer and Fluid in SD-OCT images using Mutex dice loss based fully Convolutional Networks. IEEE Access 8:60929–60939. https://doi.org/10.1109/ACCESS.2020.2983818
Dodo BI, Li Y, Kaba D, Liu X (2019) Retinal layer segmentation in Optical Coherence Tomography images. IEEE Access 7:152388–152398. https://doi.org/10.1109/ACCESS.2019.2947761
Gao Z, Wang X, Li Y (2020) Automatic Segmentation of Macular Edema in Retinal OCT Images Using Improved U-Net++. Appl Sci 10(16):5701. https://doi.org/10.3390/app10165701
Cazanas-Gordon A, Da Silva Cruz LA (2022) Multiscale Attention Gated Network (MAGNet) for retinal layer and Macular Cystoid Edema Segmentation. IEEE Access 10:85905–85917. https://doi.org/10.1109/ACCESS.2022.3198657
Ronneberger O, Fischer P, Brox T (2015) U-Net: Convolutional Networks for Biomedical Image Segmentation. ar**v May 18. https://doi.org/10.48550/ar**v.1505.04597
Zhou Z, Siddiquee MMR, Tajbakhsh N, Liang J (2018) UNet++: A Nested U-Net Architecture for Medical Image Segmentation. Deep Learn Med Image Anal Multimodal Learn Clin Decis Support 11045:3–11. https://doi.org/10.1007/978-3-030-00889-5_1
Zhang Y et al (Oct. 2021) Brain tumor segmentation from multi-modal MR images via Ensembling UNets. Front Radio 1:704888. https://doi.org/10.3389/fradi.2021.704888
Huang H et al (2020) UNet 3+: A Full-Scale Connected UNet for Medical Image Segmentation. In ICASSP 2020–2020 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), pp. 1055–1059. https://doi.org/10.1109/ICASSP40776.2020.9053405
Enokiya Y, Iwamoto Y, Chen Y-W, Han X-H (2019) Automatic Liver Segmentation using U-Net with Wasserstein GANs. J Image Graphics 7:94–101. https://doi.org/10.18178/joig.7.3.94-101
Yang D et al (2017) Automatic Liver Segmentation using an adversarial image-to-Image Network’. ar**v. Jul 25. https://doi.org/10.48550/ar**v.1707.08037
Park T, Liu M-Y, Wang T-C, Zhu J-Y (2019) Semantic Image Synthesis with Spatially-Adaptive Normalization’. ar**v. Accessed: 05 June 2023. http://arxiv.org/abs/1903.07291
Wu T, Tang S, Zhang R, Zhang Y (2019) CGNet: A Light-weight Context Guided Network for Semantic Segmentation. ar**v. Accessed: 05 June 2023. http://arxiv.org/abs/1811.08201
Lee C-Y, **e S, Gallagher P, Zhang Z, Tu Z (2015) Deeply-Supervised Nets. In: Proceedings of the Eighteenth International Conference on Artificial Intelligence and Statistics, PMLR, pp. 562–570. Accessed: 05 June 2023. https://proceedings.mlr.press/v38/lee15a.html
Lin T-Y, Goyal P, Girshick R, He K, Dollár P (2018) Focal loss for dense object detection. ar**v Feb 07. https://doi.org/10.48550/ar**v.1708.02002
He K, Zhang X, Ren S, Sun J (2015) Deep Residual Learn Image Recognition. https://doi.org/10.48550/ARXIV.1512.03385
Howard AG et al (2017) MobileNets: efficient convolutional neural networks for Mobile Vision Applications. ar**v Apr 16. https://doi.org/10.48550/ar**v.1704.04861
Schlegl T et al (2018) Fully Automated Detection and Quantification of Macular Fluid in OCT Using Deep Learning. Ophthalmology 125(4):549–558. https://doi.org/10.1016/j.ophtha.2017.10.031
Li J et al (2021) Multi-scale GCN-assisted two-stage network for joint segmentation of retinal layers and discs in peripapillary OCT images. Biomed Opt Express 12(4):2204. https://doi.org/10.1364/BOE.417212
Shi P, Qiu J, Abaxi SMD, Wei H, Lo FP-W, Yuan W (2023) Generalist Vision Foundation Models for Medical Imaging: A Case Study of Segment Anything Model on Zero-Shot Medical Segmentation. https://doi.org/10.48550/ARXIV.2304.12637
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Daanouni, O., Cherradi, B. & Tmiri, A. Automated end-to-end Architecture for Retinal Layers and Fluids Segmentation on OCT B-scans. Multimed Tools Appl (2024). https://doi.org/10.1007/s11042-024-19514-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11042-024-19514-z