Abstract
Preeclampsia (PE) is associated with high maternal and perinatal morbidity and mortality. The development of effective treatment strategies remains a major challenge due to the limited understanding of the pathogenesis. In this review, we summarize the current understanding of PE research, focusing on the molecular basis of mitochondrial function in normal and PE placentas, and discuss perspectives on future research directions. Mitochondria integrate numerous physiological processes such as energy production, cellular redox homeostasis, mitochondrial dynamics, and mitophagy, a selective autophagic clearance of damaged or dysfunctional mitochondria. Normal placental mitochondria have evolved innovative survival strategies to cope with uncertain environments (e.g., hypoxia and nutrient starvation). Cytotrophoblasts, extravillous trophoblast cells, and syncytiotrophoblasts all have distinct mitochondrial morphology and function. Recent advances in molecular studies on the spatial and temporal changes in normal mitochondrial function are providing valuable insight into PE pathogenesis. In PE placentas, hypoxia-mediated mitochondrial fission may induce activation of mitophagy machinery, leading to increased mitochondrial fragmentation and placental tissue damage over time. Repair mechanisms in mitochondrial function restore placental function, but disruption of compensatory mechanisms can induce apoptotic death of trophoblast cells. Additionally, molecular markers associated with repair or compensatory mechanisms that may influence the development and progression of PE are beginning to be identified. However, contradictory results have been obtained regarding some of the molecules that control mitochondrial biogenesis, dynamics, and mitophagy in PE placentas. In conclusion, understanding how the mitochondrial morphology and function influence cell fate decisions of trophoblast cells is an important issue in normal as well as pathological placentation biology. Research focusing on mitochondrial function will become increasingly important for elucidating the pathogenesis and effective treatment strategies of PE.


Similar content being viewed by others
Data availability
No new data were created.
References
Tranquilli AL, Dekker G, Magee L, Roberts J, Sibai BM, Steyn W, Zeeman GG, Brown MA (2014) The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens 4(2):97–104. https://doi.org/10.1016/j.preghy.2014.02.001
Staff AC (2019) The two-stage placental model of preeclampsia: an update. J Reprod Immunol 134–135:1–10. https://doi.org/10.1016/j.jri.2019.07.004
Tomimatsu T, Mimura K, Endo M, Kumasawa K, Kimura T (2017) Pathophysiology of preeclampsia: an angiogenic imbalance and long-lasting systemic vascular dysfunction. Hypertens Res 40(4):305–310. https://doi.org/10.1038/hr.2016.152
Garrido-Gomez T, Dominguez F, Quiñonero A, Diaz-Gimeno P, Kapidzic M, Gormley M, Ona K, Padilla-Iserte P, McMaster M, Genbacev O, Perales A, Fisher SJ, Simón C (2017) Defective decidualization during and after severe preeclampsia reveals a possible maternal contribution to the etiology. Proc Natl Acad Sci U S A 114(40):E8468–E8477. https://doi.org/10.1073/pnas.1706546114
Redman CWG, Staff AC, Roberts JM (2022) Syncytiotrophoblast stress in preeclampsia: the convergence point for multiple pathways. Am J Obstet Gynecol 226(2S):S907–S927. https://doi.org/10.1016/j.ajog.2020.09.047
Archibald JM (2015) Endosymbiosis and eukaryotic cell evolution. Curr Biol 25R911–25R921. https://doi.org/10.1016/j.cub.2015.07.055
Kondadi AK, Anand R, Reichert AS (2019) Functional interplay between Cristae Biogenesis, Mitochondrial Dynamics and mitochondrial DNA Integrity. Int J Mol Sci 204311. https://doi.org/10.3390/ijms20174311
Ma Y, Wang L, Jia R (2020) The role of mitochondrial dynamics in human cancers. Am J Cancer Res 10:1278–1293 eCollection 2020
Marín R, Chiarello DI, Abad C, Rojas D, Toledo F, Sobrevia L (2020) Oxidative stress and mitochondrial dysfunction in early-onset and late-onset preeclampsia. Biochim Biophys Acta Mol Basis Dis 1866(12):165961. https://doi.org/10.1016/j.bbadis.2020.165961
Zhou X, Han TL, Chen H, Baker PN, Qi H, Zhang H (2017) Impaired mitochondrial fusion, autophagy, biogenesis and dysregulated lipid metabolism is associated with preeclampsia. Exp Cell Res 359(1):195–204. https://doi.org/10.1016/j.yexcr.2017.07.029
Annesley SJ, Fisher PR (2019) Mitochondria in Health and Disease. Cells 8(7):680. https://doi.org/10.3390/cells8070680
Furui T, Kurauchi O, Tanaka M, Mizutani S, Ozawa T, Tomoda Y (1994) Decrease in cytochrome c oxidase and cytochrome oxidase subunit I messenger RNA levels in preeclamptic pregnancies. Obstet Gynecol 84(2):283–288
Oh SY, Choi SJ, Kim KH, Cho EY, Kim JH, Roh CR (2008) Autophagy-related proteins, LC3 and Beclin-1, in placentas from pregnancies complicated by preeclampsia. Reprod Sci 15(9):912–920. https://doi.org/10.1177/1933719108319159
Kalkat M, Garcia J, Ebrahimi J, Melland-Smith M, Todros T, Post M, Caniggia I (2013) Placental autophagy regulation by the BOK-MCL1 rheostat. Autophagy 9(12):2140–2153. https://doi.org/10.4161/auto.26452
Vishnyakova PA, Volodina MA, Tarasova NV, Marey MV, Kan NE, Khodzhaeva ZS, Vysokikh MY, Sukhikh GT (2017) Alterations in antioxidant system, mitochondrial biogenesis and autophagy in preeclamptic myometrium. BBA Clin 8:35–42. https://doi.org/10.1016/j.bbacli.2017.06.002
Xu P, Zheng Y, Liao J, Hu M, Yang Y, Zhang B, Kilby MD, Fu H, Liu Y, Zhang F, **ong L, Liu X, ** H, Wu Y, Huang J, Han T, Wen L, Gao R, Fu Y, Fan X, Qi H, Baker PN, Tong C (2023) AMPK regulates homeostasis of invasion and viability in trophoblasts by redirecting glucose metabolism: implications for pre-eclampsia. Cell Prolif 56(2):e13358. https://doi.org/10.1111/cpr.13358
Jahan F, Vasam G, Green AE, Bainbridge SA, Menzies KJ, Jahan F (2023) Vasam GPlacental mitochondrial function and dysfunction in Preeclampsia. Int J Mol Sci 24(4):4177. https://doi.org/10.3390/ijms24044177
Zhou X, Zhao X, Zhou W, Qi H, Zhang H, Han TL, Baker P (2021) Impaired placental mitophagy and oxidative stress are associated with dysregulated BNIP3 in preeclampsia. Sci Rep 11(1):20469. https://doi.org/10.1038/s41598-021-99837-1
Nakashima A, Aoki A, Kusabiraki T, Cheng SB, Sharma S, Saito S (2017) Autophagy regulation in preeclampsia: pros and cons. J Reprod Immunol 123:17–23. https://doi.org/10.1016/j.jri.2017.08.006
Fisher JJ, McKeating DR, Cuffe JS, Bianco-Miotto T, Holland OJ, Perkins AV (2019) Proteomic Analysis of Placental Mitochondria following trophoblast differentiation. Front Physiol 10:1536. https://doi.org/10.3389/fphys.2019.01536
Manna S, McCarthy C, McCarthy FP (2019) Placental ageing in adverse pregnancy outcomes: Telomere Shortening, Cell Senescence, and mitochondrial dysfunction. Oxid Med Cell Longev 2019:3095383. https://doi.org/10.1155/2019/3095383
Bartho LA, McKeating DR, Hannan NJ, Kaitu’u-Lino TJ, Perkins AV (2022) Transcriptional profiles of genes related to mitochondrial aging in placental pathologies. Mol Hum Reprod 29(9):gaac026. https://doi.org/10.1093/molehr/gaac026
Martínez F, Kiriakidou M, Strauss JF 3rd (1997) Structural and functional changes in mitochondria associated with trophoblast differentiation: methods to isolate enriched preparations of syncytiotrophoblast mitochondria. Endocrinology 138(5):2172–2183. https://doi.org/10.1210/endo.138.5.5133
Scorrano L (2007) Multiple functions of mitochondria-sha** proteins. Novartis Found Symp 287:47–55. https://doi.org/10.1002/9780470725207.ch4. discussion 55 – 9
Ausman J, Abbade J, Ermini L, Farrell A, Tagliaferro A, Post M, Caniggia I (2018) Ceramide-induced BOK promotes mitochondrial fission in preeclampsia. Cell Death Dis 9(3):298. https://doi.org/10.1038/s41419-018-0360-0
Westermann B (2010) Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol 11(12):872–884. https://doi.org/10.1038/nrm3013
Yu SB, Pekkurnaz G (2018) Mechanisms Orchestrating Mitochondrial Dynamics for Energy Homeostasis. J Mol Biol 430(21):3922–3941. https://doi.org/10.1016/j.jmb.2018.07.027
Abdullah MO, Zeng RX, Margerum CL, Papadopoli D, Monnin C, Punter KB, Chu C, Al-Rofaidi M, Al-Tannak NF, Berardi D, Rattray Z, Rattray NJW, Abraham SA, Eskelinen EL, Watson DG, Avizonis D, Topisirovic I, Chan EYW (2022) Mitochondrial hyperfusion via metabolic sensing of regulatory amino acids. Cell Rep 40(7):111198. https://doi.org/10.1016/j.celrep.2022.111198
Gillmore T, Farrell A, Alahari S, Sallais J, Kurt M, Park C, Ausman J, Litvack M, Post M, Caniggia I (2022) Dichotomy in hypoxia-induced mitochondrial fission in placental mesenchymal cells during development and preeclampsia: consequences for trophoblast mitochondrial homeostasis. Cell Death Dis 13(2):191. https://doi.org/10.1038/s41419-022-04641-y
Zhao J, Zhang J, Yu M, **e Y, Huang Y, Wolff DW, Abel PW, Tu Y (2013) Mitochondrial dynamics regulates migration and invasion of breast cancer cells. Oncogene 32(40):4814–4824. https://doi.org/10.1038/onc.2012.494
Bustamante J, Ramírez-Vélez R, Czerniczyniec A, Cicerchia D, Aguilar de Plata AC, Lores-Arnaiz S (2014) Oxygen metabolism in human placenta mitochondria. J Bioenerg Biomembr 46(6):459–469. https://doi.org/10.1007/s10863-014-9572-x
Adebayo M, Singh S, Singh AP, Dasgupta S (2021) Mitochondrial fusion and fission: the fine-tune balance for cellular homeostasis. FASEB J 35(6):e21620. https://doi.org/10.1096/fj.202100067R
De Castillo losR, Zarco-Zavala D, Olvera-Sanchez M, Pardo S, Juarez JP, Martinez O, Mendoza-Hernandez F, García-Trejo G, Flores-Herrera JJ (2011) Atypical cristae morphology of human syncytiotrophoblast mitochondria: role for complex V. J Biol Chem 286(27):23911–23919. https://doi.org/10.1074/jbc.M111.252056
Kolahi KS, Valent AM, Thornburg KL, Cytotrophoblast (2017) Not Syncytiotrophoblast, dominates glycolysis and oxidative phosphorylation in Human Term Placenta. Sci Rep 7:42941. https://doi.org/10.1038/srep42941
Walker OS, Gurm H, Sharma R, Verma N, May LL, Raha S (2021) Delta-9-tetrahydrocannabinol inhibits invasion of HTR8/SVneo human extravillous trophoblast cells and negatively impacts mitochondrial function. Sci Rep 11(1):4029. https://doi.org/10.1038/s41598-021-83563-9
Long J, Huang Y, Tang Z, Shan Y, Feng D, Wang W, Liu J, Huang Y, Gu H, Guo D, Yao R, Ni X (2022) Mitochondria targeted antioxidant significantly alleviates Preeclampsia caused by 11β-HSD2 dysfunction via OPA1 and MtDNA maintenance. Antioxid (Basel) 11(8):1505. https://doi.org/10.3390/antiox11081505
Vangrieken P, Al-Nasiry S, Bast A, Leermakers PA, Tulen CBM, Janssen GMJ, Kaminski I, Geomini I, Lemmens T, Schiffers PMH, van Schooten FJ, Remels AHV (2021) Hypoxia-induced mitochondrial abnormalities in cells of the placenta. PLoS ONE 16(1):e0245155. https://doi.org/10.1371/journal.pone.0245155
Kim DY, Jung SY, Kim YJ, Kang S, Park JH, Ji ST, Jang WB, Lamichane S, Lamichane BD, Chae YC, Lee D, Chung JS, Kwon SM (2018) Hypoxia-dependent mitochondrial fission regulates endothelial progenitor cell migration, invasion, and tube formation. Korean J Physiol Pharmacol 22(2):203–213. https://doi.org/10.4196/kjpp.2018.22.2.203
Arimoto-Ishida E, Sakata M, Sawada K, Nakayama M, Nishimoto F, Mabuchi S, Takeda T, Yamamoto T, Isobe A, Okamoto Y, Lengyel E, Suehara N, Morishige K, Kimura T (2009) Up-regulation of alpha5-integrin by E-cadherin loss in hypoxia and its key role in the migration of extravillous trophoblast cells during early implantation. Endocrinology 150(9):4306–4315. https://doi.org/10.1210/en.2008-1662
Cox LS, Redman C (2017) The role of cellular senescence in ageing of the placenta. Placenta 52:139–145. https://doi.org/10.1016/j.placenta.2017.01.116
Hu X-Q, Zhang L (2022) Mitochondrial dysfunction in the pathogenesis of Preeclampsia. Curr Hypertens Rep 24(6):157–172. https://doi.org/10.1007/s11906-022-01184-7Epub 2022 Mar 7
Correia Y, Scheel J, Gupta S, Wang K (2021) Placental mitochondrial function as a driver of angiogenesis and placental dysfunction. Biol Chem 402(8):887–909. https://doi.org/10.1515/hsz-2021-0121
Smith AN, Wang X, Thomas DG, Tatum RE, Booz GW, Cunningham MW (2021) The role of mitochondrial dysfunction in Preeclampsia: causative factor or collateral damage? Am J Hypertens 34(5):442–452. https://doi.org/10.1093/ajh/hpab003
Xu Z, ** X, Cai W, Zhou M, Shao P, Yang Z, Fu R, Cao J, Liu Y, Yu F, Fan R, Zhang Y, Zou S, Zhou X, Yang N, Chen X, Li Y (2018) Proteomics Analysis Reveals Abnormal Electron Transport and excessive oxidative stress cause mitochondrial dysfunction in placental tissues of early-onset Preeclampsia. Proteom Clin Appl 12(5):e1700165. https://doi.org/10.1002/prca.201700165
Vishnyakova PA, Volodina MA, Tarasova NV, Marey MV, Tsvirkun DV, Vavina OV, Khodzhaeva ZS, Kan NE, Menon R, Vysokikh MY, Sukhikh GT (2016) Mitochondrial role in adaptive response to stress conditions in preeclampsia. Sci Rep 6:32410. https://doi.org/10.1038/srep32410
He L, Wang Z, Sun Y (2004) Reduced amount of cytochrome c oxidase subunit I messenger RNA in placentas from pregnancies complicated by preeclampsia. Acta Obstet Gynecol Scand 83(2):144–148. https://doi.org/10.1111/j.0001-6349.2004.00345.x
Chen G, Lin Y, Chen L, Zeng F, Zhang L, Huang Y, Huang P, Liao L, Yu Y (2020) Role of DRAM1 in mitophagy contributes to preeclampsia regulation in mice. Mol Med Rep 22(3):1847–1858. https://doi.org/10.3892/mmr.2020.11269
Holland OJ, Cuffe JSM, Dekker Nitert M, Callaway L, Kwan Cheung KA, Radenkovic F, Perkins AV (2018) Placental mitochondrial adaptations in preeclampsia associated with progression to term delivery. Cell Death Dis 9(12):1150. https://doi.org/10.1038/s41419-018-1190-9
Beyramzadeh M, Dikmen ZG, Erturk NK, Tuncer ZS, Akbiyik F (2017) Placental respiratory chain complex activities in high risk pregnancies. J Matern Fetal Neonatal Med 30(24):2911–2917. https://doi.org/10.1080/14767058.2016.1268594
Chhimpa N, Singh N, Puri N, Kayath HP (2023) The Novel role of mitochondrial citrate synthase and citrate in the pathophysiology of Alzheimer’s Disease. J Alzheimers Dis 94(s1):S453–S472. https://doi.org/10.3233/JAD-220514
Liu X, Zuo R, Bao Y, Qu X, Sun K, Ying H (2017) Down-regulation of PDK4 is critical for the switch of Carbohydrate catabolism during syncytialization of human placental trophoblasts. Sci Rep 7(1):8474. https://doi.org/10.1038/s41598-017-09163-8
Dewi S, Triatmono VR, Rasyada Ralas PR, Veraldi V, Alfian M, Iswanti I, Prijanti FC (2022) Increasing of LDH Specific Activity and PEPCK Level play a role on activation of Gluconeogenesis Pathway in early onset Pre-eeclampsia Placenta. Rep Biochem Mol Biol 11(2):320–326. https://doi.org/10.52547/rbmb.11.2.320
Waker CA, Albers RE, Pye RL, Doliboa SR, Wyatt CN, Brown TL, Mayes DA (2017) AMPK Knockdown in placental labyrinthine progenitor cells results in restriction of critical Energy resources and terminal differentiation failure. Stem Cells Dev 26(11):808–817. https://doi.org/10.1089/scd.2016.0252
Herzig S, Shaw RJ (2018) AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol 19(2):121–135. https://doi.org/10.1038/nrm.2017.95
Kumagai A, Itakura A, Koya D, Kanasaki K (2018) AMP-Activated protein (AMPK) in pathophysiology of pregnancy complications. Int J Mol Sci 19(10):3076. https://doi.org/10.3390/ijms19103076
Poole LP, Macleod KF (2021) Mitophagy in tumorigenesis and metastasis. Cell Mol Life Sci 78(8):3817–3851. https://doi.org/10.1007/s00018-021-03774-1
Toyama EQ, Herzig S, Courchet J, Lewis TL Jr, Losón OC, Hellberg K, Young NP, Chen H, Polleux F, Chan DC, Shaw RJ (2016) Metabolism. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science 351(6270):275–281. https://doi.org/10.1126/science.aab4138
Shin EK, Kang HY, Yang H, Jung EM, Jeung EB (2016) The regulation of fatty acid oxidation in human preeclampsia. Reprod Sci 23(10):1422–1433. https://doi.org/10.1177/1933719116641759
Bartha JL, Visiedo F, Fernández-Deudero A, Bugatto F, Perdomo G (2012) Decreased mitochondrial fatty acid oxidation in placentas from women with preeclampsia. Placenta 33(2):132–134. https://doi.org/10.1016/j.placenta.2011.11.027
Wang Y, Walsh SW (2001 Feb-Mar) Increased superoxide generation is associated with decreased superoxide dismutase activity and mRNA expression in placental trophoblast cells in pre-eclampsia. Placenta 22(2–3):206–212. https://doi.org/10.1053/plac.2000.0608
Aouache R, Biquard L, Vaiman D, Miralles F (2018) Oxidative stress in Preeclampsia and placental diseases. Int J Mol Sci 19:1496. https://doi.org/10.3390/ijms19051496
Taravati A, Tohidi F (2018) Comprehensive analysis of oxidative stress markers and antioxidants status in preeclampsia. Taiwan J Obstet Gynecol 57(6):779–790. https://doi.org/10.1016/j.tjog.2018.10.002
Li J, Dong X, Liu JY, Gao L, Zhang WW, Huang YC, Wang Y, Wang H, Wei W, Xu DX (2024) FUNDC1-mediated mitophagy triggered by mitochondrial ROS is partially involved in 1-nitropyrene-evoked placental progesterone synthesis inhibition and intrauterine growth retardation in mice. Sci Total Environ 908:168383. https://doi.org/10.1016/j.scitotenv.2023.168383
Zhao M, Wang Y, Li L, Liu S, Wang C, Yuan Y, Yang G, Chen Y, Cheng J, Lu Y, Liu J (2021) Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics 11(4):1845–1863. https://doi.org/10.7150/thno.50905
Yan W, Zhang H, Liu P, Wang H, Liu J, Gao C, Liu Y, Lian K, Yang L, Sun L, Guo Y, Zhang L, Dong L, Lau WB, Gao E, Gao F, **ong L, Wang H, Qu Y, Tao L (2013) Impaired mitochondrial biogenesis due to dysfunctional adiponectin-AMPK-PGC-1α signaling contributing to increased vulnerability in diabetic heart. Basic Res Cardiol 108(3):329. https://doi.org/10.1007/s00395-013-0329-1
Zsengellér ZK, Rajakumar A, Hunter JT, Salahuddin S, Rana S, Stillman IE, Ananth Karumanchi S (2016) Trophoblast mitochondrial function is impaired in preeclampsia and correlates negatively with the expression of soluble fms-like tyrosine kinase 1. Pregnancy Hypertens 6(4):313–319. https://doi.org/10.1016/j.preghy.2016.06.004
Yu J, Guo X, Chen R, Feng L (2016) Downregulation of Mitofusin 2 in Placenta is related to Preeclampsia. Biomed Res Int 2016:6323086. https://doi.org/10.1155/2016/6323086
Soleymanlou N, Jurisica I, Nevo O, Ietta F, Zhang X, Zamudio S, Post M, Caniggia I (2005) Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab 90(7):4299–4308. https://doi.org/10.1210/jc.2005-0078
Sallais J, Park C, Alahari S, Porter T, Liu R, Kurt M, Farrell A, Post M, Caniggia I (2022) HIF1 inhibitor acriflavine rescues early-onset preeclampsia phenotype in mice lacking placental prolyl hydroxylase domain protein 2. JCI Insight 7(23):e158908. https://doi.org/10.1172/jci.insight.158908
Albers RE, Kaufman MR, Natale BV, Keoni C, Kulkarni-Datar K, Min S, Williams CR, Natale DRC, Brown TL (2019) Trophoblast-specific expression of Hif-1α results in Preeclampsia-Like symptoms and fetal growth restriction. Sci Rep 9(1):2742. https://doi.org/10.1038/s41598-019-39426-5
Marsboom G, Toth PT, Ryan JJ, Hong Z, Wu X, Fang YH, Thenappan T, Piao L, Zhang HJ, Pogoriler J, Chen Y, Morrow E, Weir EK, Rehman J, Archer SL (2012) Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ Res 110(11):1484–1497. https://doi.org/10.1161/CIRCRESAHA.111.263848
Caniggia I, Winter J, Lye SJ, Post M (2000 Mar-Apr) Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta 21:25–30 Suppl A:S. https://doi.org/10.1053/plac.1999.0522
**a QS, Lu FE, Wu F, Huang ZY, Dong H, Xu LJ, Gong J (2020) New role for ceramide in hypoxia and insulin resistance. World J Gastroenterol 26(18):2177–2186. https://doi.org/10.3748/wjg.v26.i18.2177
Aydogan Mathyk B, Temel Yuksel I, Tayyar A, Aslan Cetin B, Tayyar AT, Koroglu N (2020) Maternal serum mitofusin-2 levels in patients with preeclampsia: the possible role of mitochondrial dysfunction in preeclampsia. J Matern Fetal Neonatal Med 33(11):1861–1866. https://doi.org/10.1080/14767058.2018.1532497
Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J (2011) Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci U S A 108(25):10190–10195. https://doi.org/10.1073/pnas.1107402108
Zorova LD, Popkov VA, Plotnikov EY, Silachev DN, Pevzner IB, Jankauskas SS, Babenko VA, Zorov SD, Balakireva AV, Juhaszova M, Sollott SJ, Zorov DB (2018) Mitochondrial membrane potential. Anal Biochem 552:50–59. https://doi.org/10.1016/j.ab.2017.07.009
Fisher JJ, Bartho LA, Perkins AV, Holland OJ (2020) Placental mitochondria and reactive oxygen species in the physiology and pathophysiology of pregnancy. Clin Exp Pharmacol Physiol 47:176–184. https://doi.org/10.1111/1440-1681.13172
Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC (2003) Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160(2):189–200. https://doi.org/10.1083/jcb.200211046
Akcora Yildiz D, Irtegun Kandemir S, Agacayak E, Deveci E (2017) Evaluation of protein levels of autophagy markers (Beclin 1 and SQSTM1/p62) and phosphorylation of cyclin E in the placenta of women with preeclampsia. Cell Mol Biol (Noisy-le-grand) 63(12):51–55. https://doi.org/10.14715/cmb/2017.63.12.12
Son JM, Sarsour EH, Kakkerla Balaraju A, Fussell J, Kalen AL, Wagner BA, Buettner GR, Goswami PC (2017) Mitofusin 1 and optic atrophy 1 shift metabolism to mitochondrial respiration during aging. Aging Cell 16(5):1136–1145. https://doi.org/10.1111/acel.12649
Cushen SC, Ricci CA, Bradshaw JL, Silzer T, Blessing A, Sun J, Zhou Z, Scroggins SM, Santillan MK, Santillan DA, Phillips NR, Goulopoulou S (2022) Reduced maternal circulating cell-free mitochondrial DNA is Associated with the development of Preeclampsia. J Am Heart Assoc 11(2):e021726. https://doi.org/10.1161/JAHA.121.021726
Williamson RD, McCarthy FP, Kenny LC, McCarthy CM (2019) Activation of a TLR9 mediated innate immune response in preeclampsia. Sci Rep 9(1):5920. https://doi.org/10.1038/s41598-019-42551-w
Qiu C, Hevner K, Enquobahrie DA, Williams MA (2012) A case-control study of maternal blood mitochondrial DNA copy number and preeclampsia risk. Int J Mol Epidemiol Genet 3(3):237–244
Ding WX, Yin XM (2012) Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol Chem 393(7):547–564. https://doi.org/10.1515/hsz-2012-0119
Kang D, Kim SH, Hamasaki N Mitochondrial transcription factor A (TFAM): roles in maintenance of mtDNA and cellular functions. Mitochondrion 2007 Feb-Apr ;7(1–2):39–44. https://doi.org/10.1016/j.mito.2006.11.017
Nakashima A, Cheng SB, Ikawa M, Yoshimori T, Huber WJ, Menon R, Huang Z, Fierce J, Padbury JF, Sadovsky Y, Saito S, Sharma S (2020) Evidence for lysosomal biogenesis proteome defect and impaired autophagy in preeclampsia. Autophagy 16(10):1771–1785. https://doi.org/10.1080/15548627.2019.1707494
Ivankovic D, Chau KY, Schapira AH, Gegg ME (2016) Mitochondrial and lysosomal biogenesis are activated following PINK1/parkin-mediated mitophagy. J Neurochem 136(2):388–402. https://doi.org/10.1111/jnc.13412
Zhang L, Han L, Ma R, Hou X, Yu Y, Sun S, Xu Y, Schedl T, Moley KH, Wang Q (2015) Sirt3 prevents maternal obesity-associated oxidative stress and meiotic defects in mouse oocytes. Cell Cycle 14(18):2959–2968. https://doi.org/10.1080/15384101.2015.1026517
Fu ZJ, Wang ZY, Xu L, Chen XH, Li XX, Liao WT, Ma HK, Jiang MD, Xu TT, Xu J, Shen Y, Song B, Gao PJ, Han WQ, Zhang W (2020) HIF-1α-BNIP3-mediated mitophagy in tubular cells protects against renal ischemia/reperfusion injury. Redox Biol 36:101671. https://doi.org/10.1016/j.redox.2020.101671
Ribeiro VR, Romao-Veiga M, Nunes PR, Peracoli JC, Peracoli MTS (2022) Increase of autophagy marker p62 in the placenta from pregnant women with preeclampsia. Hum Immunol 83(5):447–452. https://doi.org/10.1016/j.humimm.2022.02.005
Tekola-Ayele F, Workalemahu T, Gorfu G, Shrestha D, Tycko B, Wapner R, Zhang C, Louis GMB (2019) Sex differences in the associations of placental epigenetic aging with fetal growth. Aging 11(15):5412–5432. https://doi.org/10.18632/aging.102124
Yildirim RM, Ergun Y, Murat Basar M (2022) Mitochondrial dysfunction, Mitophagy and their correlation with Perinatal complications: Preeclampsia and Low Birth Weight. Biomedicines 10(10):2539. https://doi.org/10.3390/biomedicines10102539
Peng X, Hou R, Yang Y, Luo Z, Cao Y (2022) Current Studies of Mitochondrial Quality Control in the Preeclampsia. Front Cardiovasc Med. Feb 28:9:836111. https://doi.org/10.3389/fcvm.2022.836111. eCollection 2022
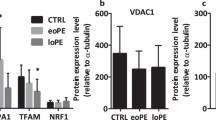
Hu H, Guo L, Overholser J, Wang X (2022) Mitochondrial VDAC1: a potential therapeutic target of inflammation-related diseases and Clinical opportunities. Cells 11(19):3174. https://doi.org/10.3390/cells11193174
Geisler S, Holmström KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W (2010) PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol 12(2):119–131. https://doi.org/10.1038/ncb2012
Tong J, Zhao W, Lv H, Li WP, Chen ZJ, Zhang C (2018) Transcriptomic profiling in human decidua of severe Preeclampsia detected by RNA sequencing. J Cell Biochem 119(1):607–615. https://doi.org/10.1002/jcb.26221
Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B (2005) Bcl-2 antiapoptotic proteins inhibit beclin 1-dependent autophagy. Cell 122(6):927–939. https://doi.org/10.1016/j.cell.2005.07.002
Sharp AN, Heazell AE, Crocker IP, Mor G (2010) Placental apoptosis in health and disease. Am J Reprod Immunol 64(3):159–169. https://doi.org/10.1111/j.1600-0897.2010.00837.x
Kasture V, Kale A, Randhir K, Sundrani D, Joshi S (2019) Effect of maternal omega-3 fatty acids and vitamin E supplementation on placental apoptotic markers in rat model of early and late onset preeclampsia. Life Sci 239:117038. https://doi.org/10.1016/j.lfs.2019.117038
Zhao L, Ma R, Zhang L, Yuan X, Wu J, He L, Liu G, Du R (2019) Inhibition of HIF-1a-mediated TLR4 activation decreases apoptosis and promotes angiogenesis of placental microvascular endothelial cells during severe pre-eclampsia pathogenesis. Placenta 83:8–16. https://doi.org/10.1016/j.placenta.2019.06.375
Wang G, Huang Y, Hu T, Zhang B, Tang Z, Yao R, Huang Y, Fan X, Ni X (2020) Contribution of placental 11β-HSD2 to the pathogenesis of preeclampsia. FASEB J 34(11):15379–15399. https://doi.org/10.1096/fj.202001003RR
Molnár M, Sütö T, Tóth T, Hertelendy F (1994) Prolonged blockade of nitric oxide synthesis in gravid rats produces sustained hypertension, proteinuria, thrombocytopenia, and intrauterine growth retardation. Am J Obstet Gynecol 170(5 pt 1):1458–1466. https://doi.org/10.1016/s0002-9378(94)70179-2
Li J, LaMarca B, Reckelhoff JF (2012) A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. Am J Physiol Heart Circ Physiol 303:H1–H8. https://doi.org/10.1152/ajpheart.00117.2012
Gatford KL, Andraweera PH, Roberts CT, Care AS (2020) Animal models of preeclampsia. Hypertension 75:1363–1381. https://doi.org/10.1161/hypertensionaha.119.14598
Xu X, Pan J-R, Zhang Y-Z (2019) CoQ10 alleviate preeclampsia symptoms by enhancing the function of mitochondria in the placenta of pregnant rats with preeclampsia. Hypertens Pregnancy 38(4):217–222. https://doi.org/10.1080/10641955.2019.1649420
Raijmakers MTM, Dechend R, Poston L (2004) Oxidative stress and preeclampsia: rationale for antioxidant clinical trials. Hypertension 44(4):374–380. https://doi.org/10.1161/01.HYP.0000141085.98320.01
Teran E, Hernandez I, Nieto B, Tavara R, Ocampo JE, Calle A (2009) Coenzyme Q10 supplementation during pregnancy reduces the risk of pre-eclampsia. Int J Gynaecol Obstet 105(1):43–45. https://doi.org/10.1016/j.ijgo.2008.11.033
Salles AM, Galvao TF, Silva MT, Motta LCD, Pereira MG (2012) Antioxidants for preventing preeclampsia: a systematic review. ScientificWorldJournal 2012:243476. https://doi.org/10.1100/2012/243476
Yang Y, Xu P, Zhu F, Liao J, Wu Y, Hu M, Fu H, Qiao J, Lin L, Huang B, ** H, Liu X, Zheng Y, Wen L, Saffery R, Kilby MD, Yan J, Kenny LC, Qi H, Tong C, Baker PN (2021) The potent antioxidant MitoQ protects against Preeclampsia during Late Gestation but increases the risk of Preeclampsia when administered in early pregnancy. Antioxid Redox Signal 34(2):118–136. https://doi.org/10.1089/ars.2019.7891
Yang Y, ** H, Qiu Y, Liu Y, Wen L, Fu Y, Qi H, Baker PN, Tong C (2022) Reactive oxygen species are essential for placental angiogenesis during early Gestation. Oxid Med Cell Longev 2022:4290922. https://doi.org/10.1155/2022/4290922
Wang J, Hansen K, Edwards R, Van Houten B, Qian W (2015) Mitochondrial division inhibitor 1 (mdivi-1) enhances death receptor-mediated apoptosis in human ovarian cancer cells. Biochem Biophys Res Commun 456(1):7–12. https://doi.org/10.1016/j.bbrc.2014.11.010
Lu M, Sferruzzi-Perri AN (2021) Placental mitochondrial function in response to gestational exposures. Placenta 104:124–137. https://doi.org/10.1016/j.placenta.2020.11.012
Chan DC (2020) Mitochondrial dynamics and its involvement in Disease. Annu Rev Pathol 15:235–259. https://doi.org/10.1146/annurev-pathmechdis-012419-032711
Nikanjam M, Kato S, Kurzrock R (2022) Liquid biopsy: current technology and clinical applications. J Hematol Oncol 15(1):131. https://doi.org/10.1186/s13045-022-01351-y
Acknowledgements
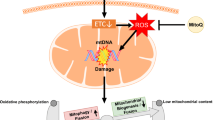
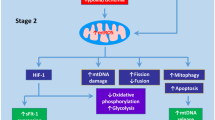
Figures were created by Toyomi Kobayashi (Ms.Clinic MayOne, Nara, Japan; https://www.mscl-mayone.com/; accessed on 9 October 31, 2023).
Author information
Authors and Affiliations
Contributions
Conception and design, H.K. Acquisition of data, S.M., C.Y., and S.I. Analysis and Interpretation of data, H.S. Drafting of the manuscript, H.K. Critical revision of the manuscript for important intellectual content, S.M., C.Y., H.S., and S.I. Statistical analysis, H.S. Administrative technical or material support, H.K. Supervision, S.I. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The submitted paper is a review article and has not been approved by the Institutional Review Board and the Research and Ethical Committee of Nara Medical University Graduate School of Medicine, Kashihara, Japan.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kobayashi, H., Yoshimoto, C., Matsubara, S. et al. An integral role of mitochondrial function in the pathophysiology of preeclampsia. Mol Biol Rep 51, 330 (2024). https://doi.org/10.1007/s11033-024-09285-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09285-z