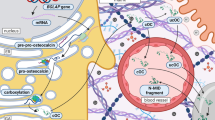
Abstract
The skeleton is a living organ that undergoes constant changes, including bone formation and resorption. It is affected by various diseases, such as osteoporosis, osteopenia, and osteomalacia. Nowadays, several methods are applied to protect bone health, including the use of hormonal and non-hormonal medications and supplements. However, certain drugs like glucocorticoids, thiazolidinediones, heparin, anticonvulsants, chemotherapy, and proton pump inhibitors can endanger bone health and cause bone loss. New studies are exploring the use of supplements, such as conjugated linoleic acid (CLA) and glucosamine, with fewer side effects during treatment. Various mechanisms have been proposed for the effects of CLA and glucosamine on bone structure, both direct and indirect. One mechanism that deserves special attention is the regulatory effect of RANKL/RANK/OPG on bone turnover. The RANKL/RANK/OPG pathway is considered a motive for osteoclast maturation and bone resorption. The cytokine system, consisting of the receptor activator of the nuclear factor (NF)-kB ligand (RANKL), its receptor RANK, and its decoy receptor, osteoprotegerin (OPG), plays a vital role in bone turnover. Over the past few years, researchers have observed the impact of CLA and glucosamine on the RANKL/RANK/OPG mechanism of bone turnover. However, no comprehensive study has been published on these supplements and their mechanism. To address this gap in knowledge, we have critically reviewed their potential effects. This review aims to assist in develo** efficient treatment strategies and focusing future studies on these supplements.

Similar content being viewed by others
Data Availability
Not applicable.
References
Szulc P (2018) Bone turnover: Biology and assessment tools. Best Pract Res Clin Endocrinol Metab 32(5):725–738. https://doi.org/10.1016/j.beem.2018.05.003
Iwaniec UT, Turner RT (2016) Influence of body weight on bone mass, architecture and turnover. J Endocrinol 230(3):R115–130. https://doi.org/10.1530/joe-16-0089
Datta HK, Ng WF, Walker JA, Tuck SP, Varanasi SS (2008) The cell biology of bone metabolism. J Clin Pathol 61(5):577–587. https://doi.org/10.1136/jcp.2007.048868
Albdeery A, Alzamily A (2022) Evaluation of the effect of injections of both platelet-rich plasma and hyaluronic acid in patients with early knee osteoarthritis via the concentration of interleukin-1β in serum. J Biomed Biochem 1(3):39–49. https://doi.org/10.57238/jbb.2022.20103
Sözen T, Özışık L, Başaran NÇ (2017) An overview and management of osteoporosis. Eur J Rheumatol 4(1):46
Banu J, Bhattacharya A, Rahman M, O’Shea M, Fernandes G (2006) Effects of conjugated linoleic acid and exercise on bone mass in young male Balb/C mice. Lipids Health Dis 5(1):7
Martinez de Victoria E (2016) Calcium, essential for health. Nutr Hosp 33(Suppl 4):341. https://doi.org/10.20960/nh.341
Corwin RL (2003) Effects of dietary fats on bone health in advanced age. Prostaglandins, leukotrienes, and essential fatty acids 68. 6379–386. https://doi.org/10.1016/s0952-3278(03)00062-0
Zhang B, **e Y, Ni Z, Chen L (2020) Effects and Mechanisms of Exogenous Electromagnetic Field on Bone cells: a review. Bioelectromagnetics. https://doi.org/10.1002/bem.22258
Pizones J, Plotkin H, Parra-Garcia JI, Alvarez P, Gutierrez P, Bueno A, Fernandez-Arroyo A (2005) Bone healing in children with osteogenesis imperfecta treated with bisphosphonates. J Pediatr Orthop 25(3):332–335
Ubios A, Furno GJ, Guglielmotti M (1991) Effect of calcitonin on alveolar wound healing. J oral Pathol Med 20(7):322–324
Grasser WA, Pan LC, Thompson DD, Paralkar VM (1997) Common mechanism for the estrogen agonist and antagonist activities of droloxifene. J Cell Biochem 65(2):159–171. https://doi.org/10.1002/(sici)1097-4644(199705)65:2>159::aid-jcb3<3.0.co;2-t
Fischer V, Haffner-Luntzer M, Amling M, Ignatius A (2018) Calcium and vitamin D in bone fracture healing and post-traumatic bone turnover. Eur Cells Mater 35:365–385. https://doi.org/10.22203/eCM.v035a25
Panday K, Gona A, Humphrey MB (2014) Medication-induced osteoporosis: screening and treatment strategies. Therapeutic Adv Musculoskelet Disease 6(5):185–202
Komori T (2016) Cell death in chondrocytes, osteoblasts, and Osteocytes. Int J Mol Sci 17(12). https://doi.org/10.3390/ijms17122045
Ono T, Nakashima T (2018) Recent advances in osteoclast biology. Histochem Cell Biol 149(4):325–341. https://doi.org/10.1007/s00418-018-1636-2
Ohlsson C, Sjogren K (2015) Effects of the gut microbiota on bone mass. Trends Endocrinol Metab 26(2):69–74. https://doi.org/10.1016/j.tem.2014.11.004
Bolamperti S, Villa I, Rubinacci A (2022) Bone remodeling: an operational process ensuring survival and bone mechanical competence. Bone Res 10(1):48
Kim JH, Liu X, Wang J, Chen X, Zhang H, Kim SH, Cui J, Li R, Zhang W, Kong Y (2013) Wnt signaling in bone formation and its therapeutic potential for bone diseases. Therapeutic Adv Musculoskelet Disease 5(1):13–31
Yu Y, Wang L, Ni S, Li D, Liu J, Chu HY, Zhang N, Sun M, Li N, Ren Q (2022) Targeting loop3 of sclerostin preserves its cardiovascular protective action and promotes bone formation. Nat Commun 13(1):4241
Wang L, Yu Y, Ni S, Li D, Liu J, **e D, Chu HY, Ren Q, Zhong C, Zhang N (2022) Therapeutic aptamer targeting sclerostin loop3 for promoting bone formation without increasing cardiovascular risk in osteogenesis imperfecta mice. Theranostics 12(13):5645
Boyce BF, **ng L (2008) Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys 473(2):139–146. https://doi.org/10.1016/j.abb.2008.03.018
Tobeiha M, Moghadasian MH, Amin N, Jafarnejad S (2020) RANKL/RANK/OPG pathway: a mechanism involved in exercise-induced bone remodeling. BioMed research international 2020
Amin N, Boccardi V, Taghizadeh M, Jafarnejad S (2020) Probiotics and bone disorders: the role of RANKL/RANK/OPG pathway. Aging Clin Exp Res 32(3):363–371
Guo Q, Li T, Qu Y, Liang M, Ha Y, Zhang Y, Wang Q (2022) New research development on trans fatty acids in food: Biological effects, analytical methods, formation mechanism, and mitigating measures. Progress in lipid research:101199
Ing SW, Belury MA (2011) Impact of conjugated linoleic acid on bone physiology: proposed mechanism involving inhibition of adipogenesis. Nutr Rev 69(3):123–131. https://doi.org/10.1111/j.1753-4887.2011.00376.x
Benjamin S, Prakasan P, Sreedharan S, Wright AD, Spener F (2015) Pros and cons of CLA consumption: an insight from clinical evidences. Nutr Metabolism 12:4. https://doi.org/10.1186/1743-7075-12-4
Terasawa N, Okamoto K, Nakada K, Masuda K (2017) Effect of conjugated linoleic acid intake on endurance Exercise Performance and Anti-fatigue in Student athletes. J Oleo Sci 66(7):723–733. https://doi.org/10.5650/jos.ess17053
Baghi AN, Mazani M, Nemati A, Amani M, Alamolhoda S, Mogadam RA (2016) Anti-inflammatory effects of conjugated linoleic acid on young athletic males. JPMA The Journal of the Pakistan Medical Association 66(3):280–284
Kirkham S, Samarasinghe R (2009) Glucosamine. J Orthop Surg 17(1):72–76
Kantor ED, Lampe JW, Navarro SL, Song X, Milne GL, White E (2014) Associations between glucosamine and chondroitin supplement use and biomarkers of systemic inflammation. J Altern Complement Med 20(6):479–485
Nagaoka I, Tsuruta A, Yoshimura M (2019) Chondroprotective action of glucosamine, a chitosan monomer, on the joint health of athletes. Int J Biol Macromol 132:795–800. https://doi.org/10.1016/j.ijbiomac.2019.03.234
Kerksick CM, Wilborn CD, Roberts MD, Smith-Ryan A, Kleiner SM, Jäger R, Collins R, Cooke M, Davis JN, Galvan E (2018) ISSN exercise & sports nutrition review update: research & recommendations. J Int Soc Sports Nutr 15(1):38
Jiang Z, Li Z, Zhang W, Yang Y, Han B, Liu W, Peng Y (2018) Dietary natural N-Acetyl-d-glucosamine prevents bone loss in ovariectomized rat model of postmenopausal osteoporosis. Molecules 23(9):2302
Tat SK, Pelletier JP, Vergés J, Lajeunesse D, Montell E, Fahmi H, Lavigne M, Martel-Pelletier J (2007) Chondroitin and glucosamine sulfate in combination decrease the pro-resorptive properties of human osteoarthritis subchondral bone osteoblasts: a basic science study. Arthritis Res Therapy 9(6):R117. https://doi.org/10.1186/ar2325
Rahman MM, Halade GV, Williams PJ, Fernandes G (2011) t10c12-CLA maintains higher bone mineral density during aging by modulating osteoclastogenesis and bone marrow adiposity. J Cell Physiol 226(9):2406–2414
Lehnen TE, da Silva MR, Camacho A, Marcadenti A, Lehnen AM (2015) A review on effects of conjugated linoleic fatty acid (CLA) upon body composition and energetic metabolism. J Int Soc Sports Nutr 12:36. https://doi.org/10.1186/s12970-015-0097-4
Kim Y, Kim J, Whang KY, Park Y (2016) Impact of conjugated linoleic acid (CLA) on skeletal muscle metabolism. Lipids 51(2):159–178. https://doi.org/10.1007/s11745-015-4115-8
Gangidi RR, Lokesh BR (2014) Conjugated linoleic acid (CLA) formation in edible oils by photoisomerization: a review. J Food Sci 79(5):R781–785. https://doi.org/10.1111/1750-3841.12449
Koronowicz AA, Banks P (2018) Antitumor Properties of CLA-Enriched Food Products. Nutr Cancer 70(4):529–545. https://doi.org/10.1080/01635581.2018.1460684
Roy BD, Antolic A (2009) Conjugated linoleic acid (CLA) and bone health: a review. Curr Top Nutraceutical Res 7(1):27
Dhiman TR, Nam SH, Ure AL (2005) Factors affecting conjugated linoleic acid content in milk and meat. Crit Rev Food Sci Nutr 45(6):463–482. https://doi.org/10.1080/10408390591034463
Kramer JK, Cruz-Hernandez C, Deng Z, Zhou J, Jahreis G, Dugan ME (2004) Analysis of conjugated linoleic acid and trans 18: 1 isomers in synthetic and animal products. Am J Clin Nutr 79(6):1137S–1145S
Kishino S, Ogawa J, Omura Y, Matsumura K, Shimizu S (2002) Conjugated linoleic acid production from linoleic acid by lactic acid bacteria. J Am Oil Chem Soc 79(2):159–163
Ogawa J, Matsumura K, Kishino S, Omura Y, Shimizu S (2001) Conjugated linoleic acid accumulation via 10-hydroxy-12-octadecaenoic acid during microaerobic transformation of linoleic acid by Lactobacillus acidophilus. Appl Environ Microbiol 67(3):1246–1252
Gorissen L, Leroy F, De Vuyst L, De Smet S, Raes K (2015) Bacterial production of conjugated linoleic and linolenic acid in foods: a technological challenge. Crit Rev Food Sci Nutr 55(11):1561–1574. https://doi.org/10.1080/10408398.2012.706243
Yang B, Gao H, Stanton C, Ross RP, Zhang H, Chen YQ, Chen H, Chen W (2017) Bacterial conjugated linoleic acid production and their applications. Prog Lipid Res 68:26–36. https://doi.org/10.1016/j.plipres.2017.09.002
Belury MA, Nickel KP, Bird CE, Wu Y (1996) Dietary conjugated linoleic acid modulation of phorbol ester skin tumor promotion
Shan Z, Luo ZP, Shen X, Chen L (2017) Promotion of fracture healing by conjugated linoleic acid in rats. J Orthop Surg 25(2):2309499017718910. https://doi.org/10.1177/2309499017718910
Pariza MW, Park Y, Cook ME (2001) The biologically active isomers of conjugated linoleic acid. Prog Lipid Res 40(4):283–298. https://doi.org/10.1016/s0163-7827(01)00008-x
Cho K, Song Y, Kwon D (2016) Conjugated linoleic acid supplementation enhances insulin sensitivity and peroxisome proliferator-activated receptor gamma and glucose transporter type 4 protein expression in the skeletal muscles of rats during endurance exercise. Iran J Basic Med Sci 19(1):20–27
Bruen R, Fitzsimons S, Belton O (2017) Atheroprotective effects of conjugated linoleic acid. Br J Clin Pharmacol 83(1):46–53. https://doi.org/10.1111/bcp.12948
Brownbill RA, Petrosian M, Ilich JZ (2005) Association between dietary conjugated linoleic acid and bone mineral density in postmenopausal women. J Am Coll Nutr 24(3):177–181
Roseti L, Desando G, Cavallo C, Petretta M, Grigolo B (2019) Articular cartilage regeneration in osteoarthritis. Cells 8(11):1305
Liu H, **ang X, Huang J, Zhu B, Wang L, Tang Y, Du F, Li L, Yan F, Ma L (2021) Ultrasound augmenting injectable chemotaxis hydrogel for articular cartilage repair in osteoarthritis. Chin Chem Lett 32(5):1759–1764
Li L, Xu H, Qu L, Nisar M, Nisar MF, Liu X, Xu K (2023) Water extracts of Polygonum Multiflorum Thunb. And its active component emodin relieves osteoarthritis by regulating cholesterol metabolism and suppressing chondrocyte inflammation. Acupunct Herb Med 3(2):96–106
Dahmer S, Schiller R (2008) Glucosamine. Am Family Phys 78(4):471–476
Dalirfardouei R, Karimi G, Jamialahmadi K (2016) Molecular mechanisms and biomedical applications of glucosamine as a potential multifunctional therapeutic agent. Life Sci 152:21–29
Matheson AJ, Perry CM (2003) Glucosamine Drugs & Aging 20(14):1041–1060
Sun S-J, Deng P, Peng C-E, Ji H-Y, Mao L-F, Peng L-Z (2022) Extraction, structure and immunoregulatory activity of low molecular weight polysaccharide from Dendrobium officinale. Polymers 14(14):2899
Wu Q, Gao Z-J, Yu X, Wang P (2022) Dietary regulation in health and disease. Signal Transduct Target Therapy 7(1):252
Huang K, Huang J, Zhao J, Gu Z, Wu J (2022) Natural lotus root-based scaffolds for bone regeneration. Chin Chem Lett 33(4):1941–1945
Epsley S, Tadros S, Farid A, Kargilis D, Mehta S, Rajapakse CS (2021) The effect of inflammation on bone. Front Physiol 11:1695
Reginster J-Y, Neuprez A, Lecart M-P, Sarlet N, Bruyere O (2012) Role of glucosamine in the treatment for osteoarthritis. Rheumatol Int 32(10):2959–2967
Kang HE, Kim SJ, Yeo E-j, Hong J, Rajgopal A, Hu C, Murray MA, Dang J, Park E (2022) Pharmacokinetic comparison of Chitosan-Derived and Biofermentation-Derived glucosamine in Nutritional supplement for Bone Health. Nutrients 14(15):3213
Williams C, Ampat G (2020) Glucosamine Sulfate
Meulyzer M, Vachon P, Beaudry F, Vinardell T, Richard H, Beauchamp G, Laverty S (2008) Comparison of pharmacokinetics of glucosamine and synovial fluid levels following administration of glucosamine sulphate or glucosamine hydrochloride. Osteoarthr Cartil 16(9):973–979. https://doi.org/10.1016/j.joca.2008.01.006
Ing SW, Belury MA (2011) Impact of conjugated linoleic acid on bone physiology: proposed mechanism involving inhibition of adipogenesis. Nutr Rev 69(3):123–131
Cusack S, Jewell C, Cashman K (2005) The effect of conjugated linoleic acid on the viability and metabolism of human osteoblast-like cells. Prostaglandins Leukot Essent Fatty Acids 72(1):29–39
Piattelli A, Scarano A, Corigliano M, Piattelli M (1996) Effects of alkaline phosphatase on bone healing around plasma-sprayed titanium implants: a pilot study in rabbits. Biomaterials 17(14):1443–1449. https://doi.org/10.1016/0142-9612(96)87288-7
Platt I, Rao LG, El-Sohemy A (2007) Isomer-specific Effects of conjugated linoleic acid on mineralized bone nodule formation from human Osteoblast-LikeCells. Experimental Biology and Medicine 232(2):246–252
Watkins BA, Shen C-L, McMurtry JP, Xu H, Bain SD, Allen KG, Seifert MF (1997) Dietary lipids modulate bone prostaglandin E2 production, insulin-like growth factor-I concentration and formation rate in chicks. J Nutr 127(6):1084–1091
Park Y, Kim J, Scrimgeour AG, Condlin ML, Kim D, Park Y (2013) Conjugated linoleic acid and calcium co-supplementation improves bone health in ovariectomised mice. Food Chem 140(1–2):280–288
Reginster JY, Neuprez A, Lecart MP, Sarlet N, Bruyere O (2012) Role of glucosamine in the treatment for osteoarthritis. Rheumatol Int 32(10):2959–2967. https://doi.org/10.1007/s00296-012-2416-2
Lv C, Wang L, Zhu X, Lin W, Chen X, Huang Z, Huang L, Yang S (2018) Glucosamine promotes osteoblast proliferation by modulating autophagy via the mammalian target of rapamycin pathway. Biomed Pharmacotherapy = Biomedecine Pharmacotherapie 99:271–277. https://doi.org/10.1016/j.biopha.2018.01.066
Henrotin Y, Chevalier X, Herrero-Beaumont G, McAlindon T, Mobasheri A, Pavelka K, Schön C, Weinans H, Biesalski H (2013) Physiological effects of oral glucosamine on joint health: current status and consensus on future research priorities. BMC Res Notes 6:115. https://doi.org/10.1186/1756-0500-6-115
Khosla S (2001) Minireview: the opg/rankl/rank system. Endocrinology 142(12):5050–5055
Hofbauer L, Kuhne C, Viereck V (2004) The OPG/RANKL/RANK system in metabolic bone diseases. J Musculoskelet Neuronal Interact 4(3):268
Wunderle M, Ruebner M, Haberle L, Schwenke E, Hack CC, Bayer CM, Koch MC, Schwitulla J, Schulz-Wendtland R, Kozieradzki I, Lux MP, Beckmann MW, Jud SM, Penninger JM, Schneider MO, Fasching PA (2020) RANKL and OPG and their influence on breast volume changes during pregnancy in healthy women. Sci Rep 10(1):5171. https://doi.org/10.1038/s41598-020-62070-3
Liu W, Zhang X (2015) Receptor activator of nuclear factor-κB ligand (RANKL)/RANK/osteoprotegerin system in bone and other tissues. Mol Med Rep 11(5):3212–3218
Kovács B, Vajda E, Nagy EE (2019) Regulatory Effects and interactions of the wnt and OPG-RANKL-RANK signaling at the bone-cartilage interface in Osteoarthritis. Int J Mol Sci 20(18). https://doi.org/10.3390/ijms20184653
Udagawa N, Koide M, Nakamura M, Nakamichi Y, Yamashita T, Uehara S, Kobayashi Y, Furuya Y, Yasuda H, Fukuda C, Tsuda E (2021) Osteoclast differentiation by RANKL and OPG signaling pathways. J Bone Miner Metab 39(1):19–26. https://doi.org/10.1007/s00774-020-01162-6
Kostenuik PJ (2005) Osteoprotegerin and RANKL regulate bone resorption, density, geometry and strength. Curr Opin Pharmacol 5(6):618–625
Dougall WC (2012) Molecular pathways: osteoclast-dependent and osteoclast-independent roles of the RANKL/RANK/OPG pathway in tumorigenesis and metastasis. Clin Cancer Res 18(2):326–335
Tu P, Duan P, Zhang R-S, Xu D-B, Wang Y, Wu H-P, Liu Y-H, Si L (2015) Polymorphisms in genes in the RANKL/RANK/OPG pathway are associated with bone mineral density at different skeletal sites in post-menopausal women. Osteoporos Int 26(1):179–185
Hsu Y-H, Niu T, Terwedow HA, Xu X, Feng Y, Li Z, Brain JD, Rosen CJ, Laird N, Xu X (2006) Variation in genes involved in the RANKL/RANK/OPG bone remodeling pathway are associated with bone mineral density at different skeletal sites in men. Hum Genet 118(5):568–577
Amin N, Clark CC, Taghizadeh M, Djafarnejad S (2020) Zinc supplements and bone health: the role of the RANKL-RANK axis as a therapeutic target. J Trace Elem Med Biol 57:126417
Rahman MM, Fernandes G, Williams P (2014) Conjugated linoleic acid prevents ovariectomy-induced bone loss in mice by modulating both osteoclastogenesis and osteoblastogenesis. Lipids 49(3):211–224. https://doi.org/10.1007/s11745-013-3872-5
Platt I, El-Sohemy A (2009) Effects of 9cis,11trans and 10trans,12cis CLA on osteoclast formation and activity from human CD14 + monocytes. Lipids Health Dis 8:15. https://doi.org/10.1186/1476-511x-8-15
Rahman MM, Bhattacharya A, Fernandes G (2006) Conjugated linoleic acid inhibits osteoclast differentiation of RAW264.7 cells by modulating RANKL signaling. J Lipid Res 47(8):1739–1748. https://doi.org/10.1194/jlr.M600151-JLR200
Sun Y, Wang C, Gong C (2020) Repairing effects of glucosamine sulfate in combination with etoricoxib on articular cartilages of patients with knee osteoarthritis. J Orthop Surg Res 15(1):150. https://doi.org/10.1186/s13018-020-01648-z
Ivanovska N, Dimitrova P (2011) Bone resorption and remodeling in murine collagenase-induced osteoarthritis after administration of glucosamine. Arthritis Res Therapy 13(2):1–13
Ali Abd Z, Jabbar N (2023) Circulating microrna-22 as a biomarker related to oxidative stress in hyperthyroid women patient. J Biomed Biochem 2(3):28–37. https://doi.org/10.57238/jbb.2023.7019.1039
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Shaymaa J. Abdulrahman, Abduladheem Turki Jalil, Mohanad Ali Abdulhadi, Dumooa Falah, Muna S. Merza, Abbas F. Almulla, Ahmed Ali and Ronak Taher Ali all contributed significantly to the development and completion of this research paper. Shaymaa J. Abdulrahman, Abduladheem Turki Jalil and Mohanad Ali Abdulhadi were responsible for conceptualizing the study and designing the research framework. Ronak Taher Ali, Dumooa Falah, and Muna S. Merza conducted the literature review, gathering relevant information on bone health, osteoporosis, and the impact of various drugs and supplements. Abbas F. Almulla and Ahmed Ali were involved in analyzing and interpreting the data related to the regulatory effect of RANKL/RANK/OPG on bone turnover concerning the use of CLA and glucosamine supplements. All authors participated in the critical review of the manuscript, providing valuable insights, and revising the content to ensure the accuracy and clarity of the information presented. Shaymaa J. Abdulrahman, Abduladheem Turki Jalil, Mohanad Ali Abdulhadi, Dumooa Falah, Muna S. Merza, Abbas F. Almulla, Ahmed Ali and Ronak Taher Ali contributed equally in the preparation of the final version of the paper, approving it for submission, and agreeing to be accountable for all aspects of the work. Their collaborative efforts have been instrumental in addressing the knowledge gap regarding the potential effects of CLA and glucosamine on the RANKL/RANK/OPG mechanism on bone turnover, thus paving the way for the development of more effective treatment strategies and guiding future studies on these supplements and their mechanisms in bone health.
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdulrahman, S., Abdulhadi, M.A., Turki Jalil, A. et al. Conjugated linoleic acid and glucosamine supplements may prevent bone loss in aging by regulating the RANKL/RANK/OPG pathway. Mol Biol Rep 50, 10579–10588 (2023). https://doi.org/10.1007/s11033-023-08839-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08839-x