Abstract
Introduction
Osteosarcoma (OS) is the most common form of bone malignancy. Although contemporary chemotherapy and surgery have improved the prognosis of those with OS, develo** new OS therapies has proven difficult for some time. The activation of the matrix metalloproteinase (MMP) and mitogen-activated protein kinase (MAPK) signaling pathways can induce metastasis, which is an obstacle to OS treatment. Ursonic acid (UNA) is a phytochemical with the potential to cure a variety of human ailments, including cancer.
Methods and results
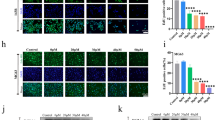
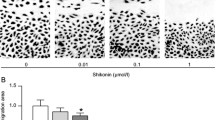
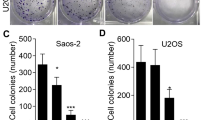
In this study, we investigated the anti-tumor properties of UNA in MG63 cells. We conducted colony formation assay, wound healing assay, and Boyden chamber assays to investigate the anti-OS effects of UNA. UNA was found to significantly inhibit the proliferative, migratory, and invasive abilities of MG63 cells. This bioactivity of UNA was mediated by the inhibition of extracellular signal-regulated kinase (ERK) and p38 and reduction of MMP-2 transcriptional expression as observed in western blot analysis, gelatin zymography and RT-PCR. Anti-OS activities of UNA were also observed in Saos2 and U2OS cells, indicating that its anti-cancer properties are not specific to cell types.
Conclusion
Our findings suggest that UNA has the potential for use in anti-metastatic drugs in the treatment of OS.






Similar content being viewed by others
Data availability
The datasets generated and analyzed during the study are available from the corresponding author on reasonable request.
Abbreviations
- ECM:
-
Extracellular matrix
- ERK:
-
Extracellular signal-regulated kinase
- JNK:
-
C-Jun N-terminal kinase
- MAPK:
-
Mitogen-activated protein kinase
- MDR:
-
Multidrug resistance
- MMP:
-
Matrix metalloproteinase
- NSCLCs:
-
Non-small-cell lung carcinomas
- OS:
-
Osteosarcoma
- PEX:
-
Hemopexin
- RT-PCR:
-
Real-time polymerase chain reaction
- ULA:
-
Ursolic acid
- UNA:
-
Ursonic acid
References
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics. CA Cancer J Clin 70(1):7–30
Ferguson JL, Turner SP (2018) Bone cancer: diagnosis and treatment principles. Am Fam Physician 98(4):205–213
Abarrategi A, Tornin J, Martinez-Cruzado L, Hamilton A, Martinez-Campos E, Rodrigo JP et al (2016) Osteosarcoma: cells-of-origin, cancer stem cells, and targeted therapies. Stem Cells Int 2016:3631764
Gill J, Gorlick R (2021) Advancing therapy for osteosarcoma. Nat Rev Clin Oncol 18(10):609–624
Harting MT, Blakely ML (2006) Management of osteosarcoma pulmonary metastases. Semin Pediatr Surg 15(1):25–29
Cui J, Dean D, Hornicek FJ, Chen Z, Duan Z (2020) The role of extracelluar matrix in osteosarcoma progression and metastasis. J Exp Clin Cancer Res 39(1):178
Lee YJ, Lee SY (2021) Maclurin exerts anti-cancer effects in human osteosarcoma cells via prooxidative activity and modulations of PARP, p38, and ERK signaling. IUBMB Life 73(8):1060–1072
Son J, Lee SY (2021) Emetine exerts anticancer effects in U2OS human osteosarcoma cells via activation of p38 and inhibition of ERK, JNK, and beta-catenin signaling pathways. J Biochem Mol Toxicol 35(10):e22868
Yang C, Zhang L, Huang H, Yuan X, Zhang P, Ye C et al (2022) Alantolactone inhibits proliferation, metastasis and promotes apoptosis of human osteosarcoma cells by suppressing Wnt/beta-catenin and MAPKs signaling pathways. Genes Dis 9(2):466–478
Son J, Lee SY (2020) Therapeutic potential of ursonic acid: Comparison with ursolic acid. Biomolecules 10(11):1505
Yang S, Zhao Q, **ang H, Liu M, Zhang Q, Xue W et al (2013) Antiproliferative activity and apoptosis-inducing mechanism of constituents from Toona sinensis on human cancer cells. Cancer Cell Int 13(1):12
Son J, Lee SY (2020) Ursonic acid exerts inhibitory effects on matrix metalloproteinases via ERK signaling pathway. Chem Biol Interact 315:108910
Liao X, Zhou X, Mak NK, Leung KN (2013) Tryptanthrin inhibits angiogenesis by targeting the VEGFR2-mediated ERK1/2 signalling pathway. PLoS ONE 8(12):e82294
Lee SI, Bae JA, Ko YS, Lee KI, Kim H, Kim KK (2016) Geijigajakyak decoction inhibits the motility and tumorigenesis of colorectal cancer cells. BMC Complement Altern Med 16(1):288
Yuan XH, Zhang P, Yu TT, Huang HK, Zhang LL, Yang CM et al (2020) Lycorine inhibits tumor growth of human osteosarcoma cells by blocking Wnt/beta-catenin, ERK1/2/MAPK and PI3K/AKT signaling pathway. Am J Transl Res 12(9):5381–5398
Chandhanayingyong C, Kim Y, Staples JR, Hahn C, Lee FY (2012) MAPK/ERK signaling in osteosarcomas, ewing sarcomas and chondrosarcomas: therapeutic implications and future directions. Sarcoma 2012:404810
Kessenbrock K, Plaks V, Werb Z (2010) Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141(1):52–67
Tallant C, Marrero A, Gomis-Ruth FX (2010) Matrix metalloproteinases: fold and function of their catalytic domains. Biochim Biophys Acta 1803:20–28
Alford VM, Kamath A, Ren X, Kumar K, Gan Q, Awwa M et al (2017) Targeting the hemopexin-like domain of latent matrix metalloproteinase-9 (proMMP-9) with a small molecule inhibitor prevents the formation of focal adhesion junctions. ACS Chem Biol 12(11):2788–2803
Okusha Y, Eguchi T, Sogawa C, Okui T, Nakano K, Okamoto K et al (2018) The intranuclear PEX domain of MMP involves proliferation, migration, and metastasis of aggressive adenocarcinoma cells. J Cell Biochem 119(9):7363–7376
Deryugina EI, Quigley JP (2006) Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev 25(1):9–34
Raeeszadeh-Sarmazdeh M, Do LD, Hritz BG (2020) Metalloproteinases and their inhibitors: potential for the development of new therapeutics. Cells 9(5):1313
Bjornland K, Flatmark K, Pettersen S, Aaasen AO, Fodstad O, Maelandsmo GM (2005) Matrix metalloproteinases participate in osteosarcoma invasion. J Surg Res 127(2):151–156
Huang JF, Du WX, Chen JJ (2016) Elevated expression of matrix metalloproteinase-3 in human osteosarcoma and its association with tumor metastasis. J BUON 21(5):1279–1286
Rubin EM, Guo Y, Tu K, **e J, Zi X, Hoang BH (2010) Wnt inhibitory factor 1 decreases tumorigenesis and metastasis in osteosarcoma. Mol Cancer Ther 9(3):731–741
Braicu C, Buse M, Busuioc C, Drula R, Gulei D, Raduly L et al (2019) A comprehensive review on MAPK: a promising therapeutic target in cancer. Cancers 11(10):1618
Lawrence MC, Jivan A, Shao C, Duan L, Goad D, Zaganjor E et al (2008) The roles of MAPKs in disease. Cell Res 18(4):436–442
Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, Hu LL (2020) ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med 19(3):1997–2007
Zhu G, Shen Q, Jiang H, Ji O, Zhu L, Zhang L (2020) Curcumin inhibited the growth and invasion of human monocytic leukaemia SHI-1 cells in vivo by altering MAPK and MMP signalling. Pharm Biol 58(1):25–34
Yao M, Wang X, Zhao Y, Wang X, Gao F (2017) Expression of MMPs is dependent on the activity of mitogen-activated protein kinase in chondrosarcoma. Mol Med Rep 15(2):915–921
Kumar B, Koul S, Petersen J, Khandrika L, Hwa JS, Meacham RB et al (2010) p38 mitogen-activated protein kinase-driven MAPKAPK2 regulates invasion of bladder cancer by modulation of MMP-2 and MMP-9 activity. Cancer Res 70(2):832–841
Cheng G, Gao F, Sun X, Bi H, Zhu Y (2016) Paris saponin VII suppresses osteosarcoma cell migration and invasion by inhibiting MMP2/9 production via the p38 MAPK signaling pathway. Mol Med Rep 14(4):3199–3205
Su Y, Wan D, Song W (2016) Dryofragin inhibits the migration and invasion of human osteosarcoma U2OS cells by suppressing MMP-2/9 and elevating TIMP-1/2 through PI3K/AKT and p38 MAPK signaling pathways. Anticancer Drugs 27(7):660–668
Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM (2006) Targeting multidrug resistance in cancer. Nat Rev Drug Discov 5(3):219–234
Hattinger CM, Patrizio MP, Fantoni L, Casotti C, Riganti C, Serra M (2021) Drug resistance in osteosarcoma: emerging biomarkers, therapeutic targets and treatment strategies. Cancers 13(12):2878
Mohanty S, Aghighi M, Yerneni K, Theruvath JL, Daldrup-Link HE (2019) Improving the efficacy of osteosarcoma therapy: combining drugs that turn cancer cell ‘don’t eat me’ signals off and ‘eat me’ signals on. Mol Oncol 13(10):2049–2061
Higuchi T, Sugisawa N, Miyake K, Oshiro H, Yamamoto N, Hayashi K et al (2019) Combination treatment with sorafenib and everolimus regresses a doxorubicin-resistant osteosarcoma in a PDOX mouse model. Anticancer Res 39(9):4781–4786
Xu C, Wang M, Guo W, Sun W, Liu Y (2021) Curcumin in osteosarcoma therapy: combining with immunotherapy, chemotherapeutics, bone tissue engineering materials and potential synergism with photodynamic therapy. Front Oncol 11:672490
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) and funded by the Ministry of Science and ICT (NRF-2022R1F1A1062695). This research was also supported by Gachon University research fund of 2021 (GCU-202104430001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Son, J., Cha, H., Lee, S. et al. Ursonic acid inhibits migration and invasion of human osteosarcoma cells through the suppression of mitogen-activated protein kinases and matrix metalloproteinases. Mol Biol Rep 50, 4029–4038 (2023). https://doi.org/10.1007/s11033-023-08333-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08333-4