Abstract
Background
Hepatitis B virus (HBV) infection is closely associated with the malignant progression of hepatocellular carcinoma (HCC). However, the mechanism involved in the HBV-related HCC development remains poorly understood. Hence, the aim of this study is to investigate the regulatory mechanism of EphA2-induced epithelial-mesenchymal transition (EMT) in the metastasis of HBV-related HCC cells.
Methods and results
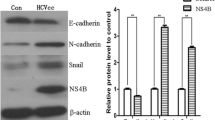
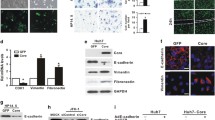
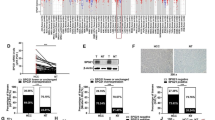
The expression level of EphA2 was determined in HBV-related human HCC cells. Then, the effects of EphA2 silencing on the EMT-associated proteins, the Wnt/β-catenin signal pathway and the metastatic potential of HBV-related HCC cells were evaluated. Finally, the inhibitory role of Entecavir (a potent antiviral drug for HBV) on EphA2-induced EMT was explored. The present study revealed that the EphA2 expression level was increased in HBV-related HCC cells compared with non-related HCC cells. Following EphA2 knockdown, the downregulation of Vimentin, β-catenin and p-GSK-3βSer9 expressions, the upregulation of E-cadherin expression, and the suppressed migration and invasion ability of HBV-related HCC cells were found. Additionally, Entecavir was proved to have a significant inhibitory effect on EphA2-induced EMT via attenuating the Wnt/β-catenin signal pathway.
Conclusions
In this study, we found that EphA2-induced EMT was involved in the enhanced metastatic potential of HBV-related HCC cells through the activation of the Wnt/β-catenin signal pathway.





Similar content being viewed by others
References
Mak LY, Wong DK, Pollicino T, Raimondo G, Hollinger FB, Yuen MF (2020) Occult hepatitis B infection and hepatocellular carcinoma: epidemiology, virology, hepatocarcinogenesis and clinical significance. J Hepatol 73:952–964. https://doi.org/10.1016/j.jhep.2020.05.042
Tan AT, Yang N, Lee Krishnamoorthy T, Oei V, Chua A, Zhao X, Tan HS, Chia A, Le Bert N, Low D, Tan HK, Kumar R, Irani FG, Ho ZZ, Zhang Q, Guccione E, Wai LE, Koh S, Hwang W, Chow WC, Bertoletti A (2019) Use of expression profiles of HBV-DNA integrated into genomes of hepatocellular carcinoma cells to select T cells for immunotherapy. Gastroenterology 156:1862–1876. https://doi.org/10.1053/j.gastro.2019.01.251
Chen Y, Zhang L, Zhang Y, Bai T, Song J, Qian W, Hou X (2020) EphrinA1/EphA2 promotes epithelial hyperpermeability involving in lipopolysaccharide-induced intestinal barrier dysfunction. J Neurogastroenterol Motil 26:397–409. https://doi.org/10.5056/jnm19095
**ao T, **ao Y, Wang W, Tang YY, **ao Z, Su M (2020) Targeting EphA2 in cancer. J Hematol Oncol 13:114. https://doi.org/10.1186/s13045-020-00944-9
Dunne PD, Dasgupta S, Blayney JK, McArt DG, Redmond KL, Weir JA, Bradley CA, Sasazuki T, Shirasawa S, Wang T, Srivastava S, Ong CW, Arthur K, Salto-Tellez M, Wilson RH, Johnston PG, Schaeybroeck SV (2016) EphA2 expression is a key driver of migration and invasion and a poor prognostic marker in colorectal cancer. Clin Cancer Res 22:230–242. https://doi.org/10.1158/1078-0432.CCR-15-0603
Baek SH, Ko JH, Lee JH, Kim C, Lee H, Nam D, Lee J, Lee SG, Yang WM, Um JY, Sethi G, Ahn KS (2017) Ginkgolic acid inhibits invasion and migration and TGF-β-induced EMT of lung cancer cells through PI3K/Akt/mTOR inactivation. J Cell Physiol 232:346–354. https://doi.org/10.1002/jcp.25426
Tanaka T, Goto K, Iino M (2016) Sec8 modulates TGF-β induced EMT by controlling N-cadherin via regulation of Smad3/4. Cell Signal 29:115–126. https://doi.org/10.1016/j.cellsig.2016.10.007
**ao C, Wu CH, Hu HZ (2016) LncRNA UCA1 promotes epithelial-mesenchymal transition (EMT) of breast cancer cells via enhancing Wnt/beta-catenin signaling pathway. Eur Rev Med Pharmacol Sci 20:2819–2824 (PMID: 27424981)
Jiang Y, Zhan H (2020) Communication between EMT and PD-L1 signaling: new insights into tumor immune evasion. Cancer Lett 468:72–81. https://doi.org/10.1016/j.canlet.2019.10.013
Fattet L, Jung HY, Matsumoto MW, Aubol BE, Kumar A, Adams JA, Chen AC, Sah RL, Engler AJ, Pasquale EB, Yang J (2020) Matrix rigidity controls epithelial-mesenchymal plasticity and tumor metastasis via a mechanoresponsive EPHA2/LYN complex. Dev Cell 54:302–316. https://doi.org/10.1016/j.devcel.2020.05.031
Hong JY, Shin MH, Douglas IS, Chung KS, Kim EY, Jung JY, Kang YA, Kim SK, Chang J, Kim YS, Park MS (2016) Inhibition of EphA2/EphrinA1 signal attenuates lipopolysaccharide- induced lung injury. Clin Sci (Lond) 130:1993–2003. https://doi.org/10.1042/CS20160360
Tsouko E, Wang J, Frigo DE, Aydoğdu E, Williams C (2015) miR-200a inhibits migration of triple-negative breast cancer cells through direct repression of the EphA2 oncogene. Carcinogenesis 36:1051–1060. https://doi.org/10.1093/carcin/bgv087
Oner E, Kotmakci M, Baird AM, Gray SG, Butuner BD, Bozkurt E, Kantarci AG, Finn SP (2021) Development of EphA2 siRNA-loaded lipid nanoparticles and combination with a small-molecule histone demethylase inhibitor in prostate cancer cells and tumor spheroids. Pharm Res 19:71. https://doi.org/10.1186/s12951-021-00781-z
Feng J, Lu SS, **ao T, Huang W, Yi H, Zhu W, Fan S, Feng XP, Li JY, Yu ZZ, Gao S, Nie GH, Tang YY, **ao ZQ (2020) ANXA1 binds and stabilizes EphA2 to promote nasopharyngeal carcinoma growth and metastasis. Cancer Res 80:4386–4398. https://doi.org/10.1158/0008-5472.CAN-20-0560
** Q, Li XJ, Cao PG (2016) miR-26b enhances radiosensitivity of hepatocellular carcinoma cells by targeting EphA2. Iran J Basic Med Sci 19:851–857 (PMID: 27746866)
Yi Z, Prinzing BL, Cao F, Gottschalk S, Krenciute G (2018) Optimizing EphA2-CAR T cells for the adoptive immunotherapy of glioma. Mol Ther Methods Clin Dev 9:70–80. https://doi.org/10.1016/j.omtm.2018.01.009
Cui XD, Lee MJ, Yu GR, Kim IH, Yu HC, Song EY, Kim DG (2010) EFNA1 ligand and its receptor EphA2: potential biomarkers for hepatocellular carcinoma. Int J Cancer 1126:940–949. https://doi.org/10.1002/ijc.24798
Levrero M, Zucman-Rossi J (2016) Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol 64:S84–S101. https://doi.org/10.1016/j.jhep.2016.02.021
Kong F, Hu W, Zhou K, Wei X, Kou Y, You H, Zheng K, Tang R (2016) Hepatitis B virus X protein promotes interleukin-7 receptor expression via NF-κB and Notch1 pathway to facilitate proliferation and migration of hepatitis B virus-related hepatoma cells. J Exp Clin Cancer Res 35:172. https://doi.org/10.1186/s13046-016-0448-2
Zhang X, Zhang T, Yang K, Zhang M, Wang K (2016) miR-486-5p suppresses prostate cancer metastasis by targeting snail and regulating epithelial-mesenchymal transition. Onco Targets Ther 9:6909–6914. https://doi.org/10.2147/OTT.S117338
Wei S, Wang L, Zhang L, Li B, Li Z, Zhang Q, Wang J, Chen L, Sun G, Li Q, Xu H, Zhang D, Xu Z (2016) ZNF143 enhances metastasis of gastric cancer by promoting the process of EMT through PI3K/AKT signaling pathway. Tumor Biol 37:12813–12821. https://doi.org/10.1007/s13277-016-5239-z
Hou F, Yuan W, Huang J, Qian L, Chen Z, Ge J, Wu S, Chen J, Wang J, Chen Z (2012) Overexpression of EphA2 correlates with epithelial-mesenchymal transition-related proteins in gastric cancer and their prognostic importance for postoperative patients. Med Oncol 29:2691–2700. https://doi.org/10.1007/s12032-011-0127-2
Zhou J, Jain S, Azad AK, Xu X, Yu HC, Xu Z, Godbout R, Fu Y (2016) Notch and TGFβ form a positive regulatory loop and regulate EMT in epithelial ovarian cancer cells. Cell Signal 28:838–849. https://doi.org/10.1016/j.cellsig.2016.03.016
Gu Y, Wang Q, Guo K, Qin W, Liao W, Wang S, Ding Y, Lin J (2016) TUSC3 promotes colorectal cancer progression and epithelial-mesenchymal transition (EMT) through WNT/β-catenin and MAPK signalling. J Pathol 239:60–71. https://doi.org/10.1002/path.4697
Nguyen VHL, Hough R, Bernaudo S, Peng C (2019) Wnt/β-catenin signalling in ovarian cancer: insights into its hyperactivation and function in tumorigenesis. J Ovarian Res 12:122. https://doi.org/10.1186/s13048-019-0596-z
He S, Tang S (2020) WNT/beta-catenin signaling in the development of liver cancers. Biomed Pharmacother 132:110851. https://doi.org/10.1016/j.biopha.2020.110851
Vilchez V, Turcios L, Marti F, Gedaly R (2016) Targeting Wnt/β-catenin pathway in hepatocellular carcinoma treatment. World J Gastroenterol 22:823–832. https://doi.org/10.3748/wjg.v22.i2.823
Bian J, Dannappel M, Wan C, Firestein R (2020) Transcriptional regulation of Wnt/beta-catenin pathway in colorectal cancer. Cells 9:2125. https://doi.org/10.3390/cells9092125
Leone P, Solimando AG, Fasano R, Argentiero A, Malerba E, Buonavoglia A, Lupo LG, De Re V, Silvestris N, Racanelli V (2021) The evolving role of immune checkpoint inhibitors in hepatocellular carcinoma treatment. Vaccines (Basel) 9:532. https://doi.org/10.3390/vaccines9050532
Solimando AG, Susca N, Argentiero A, Brunetti O, Leone P, De Re V, Fasano R, Krebs M, Petracci E, Azzali I, Nanni O, Silvestris N, Vacca A, Racanelli V (2022) Second-line treatments for advanced hepatocellular carcinoma: a systematic review and bayesian network meta-analysis. Clin Exp Med 22:65–74. https://doi.org/10.1007/s10238-021-00727-7
Acknowledgements
This work was supported by grants from the Key Project of Tian** Science and Technology Support Programs of China (Grant No. 20YFZCSY00310) and the Science Foundation of Tian** Health and Family Planning Commission of China (Grant Nos. 15KG114, TJWJ2022MS021 and 2010KY03).
Funding
The Funded was provided by the Key Project of Tian** Science and Technology Support Program of China (Grant No. 20YFZCSY00310) and the Science Foundation of Tian** Health and Family Planning Commission of China (Grant Nos. 15KG114, TJWJ2022MS021 and 2010KY03).
Author information
Authors and Affiliations
Contributions
HL and JL conceived and designed the study and all the experiments. YW, ZZ and HL performed the experiments and collected all data. ZZ, PW and JZ analyzed the data. HL and JL wrote and edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors certify that they have no financial and personal relationship that could inappropriately influence or bias this work.
Ethical approval
Not applicable.
Consent to participation
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Zhang, Z., Zhu, Z. et al. The significance of EphA2-regulated Wnt/β-catenin signal pathway in promoting the metastasis of HBV-related hepatocellular carcinoma. Mol Biol Rep 50, 565–575 (2023). https://doi.org/10.1007/s11033-022-08045-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-08045-1