Abstract
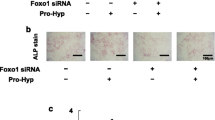
Vitexin (apigenin-8-C-d-glucopyranoside) is a flavonoid isolated from natural sources. It has been employed as an anti-oxidant, anti-inflammatory, and anti-cancer agent, and is used as a traditional Chinese medicine to treat a variety of illnesses. The present study investigated the effect of vitexin on osteoblast differentiation of C3H10T1/2 mesenchymal stem cells, MC3T3-E1 preosteoblast, mouse calvarial primary cells, and primary bone marrow stem cells (BMSCs). RT-PCR and quantitative PCR demonstrated that vitexin increased mRNA expression of the osteogenic genes distal-less homeobox 5 (Dlx5) and Runxt-related transcription factor 2 (Runx2). Vitexin also increased the Dlx5 and Runx2 protein levels, Smad1/5/9 phosphorylation, and alkaline phosphatase (ALP) activity. In addition, vitexin increased Runx2-luciferase activity. Moreover, knockdown of Runx2 attenuated the increase in ALP activity induced by vitexin. These results demonstrate that vitexin enhances osteoblast differentiation via Runx2.






Similar content being viewed by others
Abbreviations
- MSC:
-
Mesenchymal stem cell
- BMSC:
-
Bone marrow stromal cell
- BMP:
-
Bone morphogenetic proteins
- Runx2:
-
Runt-related transcription factor 2
- TNF-α:
-
Tumour necrosis factor-α
- ALP:
-
Alkaline phosphatase
- DMEM:
-
Dulbecco's Modified Eagle's Medium
- Dlx5:
-
Distal-less homeobox 5
- DMSO:
-
Dimethyl sulfoxide
- Smad:
-
Small mothers against
- FBS:
-
Fetal bovine serum
References
Olsen BR, Reginato AM, Wang W (2000) Bone development. Annu Rev Cell Dev Biol 16:191–220. https://doi.org/10.1146/annurev.cellbio.16.1.191
Abu-Amer Y, Darwech I, Clohisy JC (2007) Aseptic loosening of total joint replacements: mechanisms underlying osteolysis and potential therapies. Arthrit Res Ther 9(Suppl 1):S6. https://doi.org/10.1186/ar2170
Mbalaviele G, Novack DV, Schett G, Teitelbaum SL (2017) Inflammatory osteolysis: a conspiracy against bone. J Clin Investig 127(6):2030–2039. https://doi.org/10.1172/JCI93356
Ducy P, Karsenty G (1995) Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol Cell Biol 15(4):1858–1869. https://doi.org/10.1128/mcb.15.4.1858
Grigoriadis AE, Heersche JN, Aubin JE (1988) Differentiation of muscle, fat, cartilage, and bone from progenitor cells present in a bone-derived clonal cell population: effect of dexamethasone. J Cell Biol 106(6):2139–2151. https://doi.org/10.1083/jcb.106.6.2139
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284(5411):143–147
Li Y, Ge C, Long JP, Begun DL, Rodriguez JA, Goldstein SA, Franceschi RT (2012) Biomechanical stimulation of osteoblast gene expression requires phosphorylation of the RUNX2 transcription factor. J Bone Miner Res 27(6):1263–1274. https://doi.org/10.1002/jbmr.1574
Stein GS, Lian JB, van Wijnen AJ, Stein JL, Montecino M, Javed A, Zaidi SK, Young DW, Choi JY, Pockwinse SM (2004) Runx2 control of organization, assembly and activity of the regulatory machinery for skeletal gene expression. Oncogene 23(24):4315–4329. https://doi.org/10.1038/sj.onc.1207676
Banerjee C, McCabe LR, Choi JY, Hiebert SW, Stein JL, Stein GS, Lian JB (1997) Runt homology domain proteins in osteoblast differentiation: AML3/CBFA1 is a major component of a bone-specific complex. J Cell Biochem 66(1):1–8
Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G (1997) Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89(5):747–754. https://doi.org/10.1016/s0092-8674(00)80257-3
Harada H, Tagashira S, Fujiwara M, Ogawa S, Katsumata T, Yamaguchi A, Komori T, Nakatsuka M (1999) Cbfa1 isoforms exert functional differences in osteoblast differentiation. J Biol Chem 274(11):6972–6978. https://doi.org/10.1074/jbc.274.11.6972
He M, Min JW, Kong WL, He XH, Li JX, Peng BW (2016) A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia 115:74–85. https://doi.org/10.1016/j.fitote.2016.09.011
Ganesan K, Xu B (2017) Molecular targets of vitexin and isovitexin in cancer therapy: a critical review. Ann N Y Acad Sci 1401(1):102–113. https://doi.org/10.1111/nyas.13446
Min JW, Hu JJ, He M, Sanchez RM, Huang WX, Liu YQ, Bsoul NB, Han S, Yin J, Liu WH, He XH, Peng BW (2015) Vitexin reduces hypoxia-ischemia neonatal brain injury by the inhibition of HIF-1alpha in a rat pup model. Neuropharmacology 99:38–50. https://doi.org/10.1016/j.neuropharm.2015.07.007
Zhang Y, Bao B, Lu B, Ren Y, Tie X, Zhang Y (2005) Determination of flavone C-glucosides in antioxidant of bamboo leaves (AOB) fortified foods by reversed-phase high-performance liquid chromatography with ultraviolet diode array detection. J Chromatogr A 1065(2):177–185
Borghi SM, Carvalho TT, Staurengo-Ferrari L, Hohmann MS, **e-Filho P, Casagrande R, Verri WA Jr (2013) Vitexin inhibits inflammatory pain in mice by targeting TRPV1, oxidative stress, and cytokines. J Nat Prod 76(6):1141–1149. https://doi.org/10.1021/np400222v
Jiang J, Jia Y, Lu X, Zhang T, Zhao K, Fu Z, Pang C, Qian Y (2019) Vitexin suppresses RANKL-induced osteoclastogenesis and prevents lipopolysaccharide (LPS)-induced osteolysis. J Cell Physiol. https://doi.org/10.1002/jcp.28378
Maridas DE, Rendina-Ruedy E, Le PT, Rosen CJ (2018) Isolation, culture, and differentiation of bone marrow stromal cells and osteoclast progenitors from mice. J Vis Exp. https://doi.org/10.3791/56750
Erceg I, Tadic T, Kronenberg MS, Marijanovic I, Lichtler AC (2003) Dlx5 regulation of mouse osteoblast differentiation mediated by avian retrovirus vector. Croat Med J 44(4):407–411
Lee MH, Kim YJ, Kim HJ, Park HD, Kang AR, Kyung HM, Sung JH, Wozney JM, Kim HJ, Ryoo HM (2003) BMP-2-induced Runx2 expression is mediated by Dlx5, and TGF-beta 1 opposes the BMP-2-induced osteoblast differentiation by suppression of Dlx5 expression. J Biol Chem 278(36):34387–34394. https://doi.org/10.1074/jbc.M211386200
Wu M, Chen G, Li YP (2016) TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res 4:16009. https://doi.org/10.1038/boneres.2016.9
Rodan GA, Noda M (1991) Gene expression in osteoblastic cells. Crit Rev Eukaryot Gene Expr 1(2):85–98
Acknowledgements
This research was supported by Daegu University Research Grant 2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have a conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, KM., Son, HE., Min, HY. et al. Vitexin enhances osteoblast differentiation through phosphorylation of Smad and expression of Runx2 at in vitro and ex vivo. Mol Biol Rep 47, 8809–8817 (2020). https://doi.org/10.1007/s11033-020-05929-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05929-y