Abstract
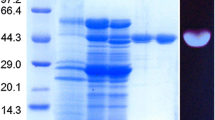
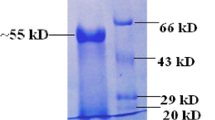
An endo-1,4-β-d-glucanase gene was cloned from the thermophilic archaea Sulfolobus shibatae and expressed in E. coli. The recombinant enzyme was purified by heat denaturation and affinity chromatography prior to characterisation. The purified recombinant enzyme exhibited maximum activity at 95–100 °C and displayed a broad pH profile with over 91% of its maximum activity observed at pH 3–5. Upon assessment of enzyme thermal stability, full activity was observed after 1 h incubation at 75, 80 and 85 °C while 98%, 90% and 84% of original activity was detected after 2 h at 75, 80 and 85 °C, respectively. Maximum activity was observed on barley β-glucan and lichenan and the purified enzyme also hydrolysed CMC and xylan. Endoglucanase activity was confirmed by viscometric assay with a rapid decrease in substrate viscosity observed immediately upon incubation with barley β-glucan or CMC. The crude enzyme released reducing sugars from acid-pretreated straw at 75–85 °C. The thermophilic nature and biochemical properties of the enzyme indicate its potential suitability in industrial applications undertaken at high temperature, such as the production of second-generation bioethanol from lignocellulosic feedstocks and in the brewing industry. This is the first known report of an endoglucanase from S. shibatae.







Similar content being viewed by others
References
Raddadi N, Cherif A, Daffonchio D, Neifar M, Fava F (2015) Biotechnological applications of extremophiles, extremozymes and extremolytes. Appl Microbiol Biotechnol 99(19):7907–7913. https://doi.org/10.1007/s00253-015-6874-9
Akram F, Haq IU, Imran W, Mukhtar H (2018) Insight perspectives of thermostable endoglucanases for bioethanol production: a review. Renewable Energy 122:225–238. https://doi.org/10.1016/j.renene.2018.01.095
Haq I, Akram F (2017) Enhanced production of a recombinant multidomain thermostable GH9 processive endo-1,4-β-glucanase (CenC) from Ruminiclostridium thermocellum in a mesophilic host through various cultivation and induction strategies. Appl Biochem Biotechnol 183(1):171–188. https://doi.org/10.1007/s12010-017-2437-0
Sharma A, Tewari R, Rana SS, Soni R, Soni SK (2016) Cellulases: classification, methods of determination and industrial applications. Appl Biochem Biotechnol 179(8):1346–1380. https://doi.org/10.1007/s12010-016-2070-3
Bhalla A, Bansal N, Kumar S, Bischoff KM, Sani RK (2013) Improved lignocellulose conversion to biofuels with thermophilic bacteria and thermostable enzymes. Bioresour Technol 128:751–759. https://doi.org/10.1016/j.biortech.2012.10.145
Dos Santos LV, De Barros Grassi MC, Gallardo JCM, Pirolla RAS, Calderón LL, De Carvalho-Netto OV, Parreiras LS, Camargo ELO, Drezza AL, Missawa SK, Teixeira GS, Lunardi I, Bressiani J, Pereira GAG (2016) Second-generation ethanol: the need is becoming a reality. Ind Biotechnol 12(1):40–57. https://doi.org/10.1089/ind.2015.0017
Kuhad RC, Deswal D, Sharma S, Bhattacharya A, Jain KK, Kaur A, Pletschke BI, Singh A, Karp M (2016) Revisiting cellulase production and redefining current strategies based on major challenges. Renewable Sustain Energy Rev 55:249–272. https://doi.org/10.1016/j.rser.2015.10.132
Irfan M, Tayyab A, Hasan F, Khan S, Badshah M, Shah AA (2017) Production and characterization of organic solvent-tolerant cellulase from Bacillus amyloliquefaciens AK9 isolated from hot spring. Appl Biochem Biotechnol 182(4):1390–1402. https://doi.org/10.1007/s12010-017-2405-8
Jain KK, Kumar S, Deswal D, Kuhad RC (2017) Improved production of thermostable cellulase from Thermoascus aurantiacus RCKK by fermentation bioprocessing and its application in the hydrolysis of office waste paper, algal pulp, and biologically treated wheat straw. Appl Biochem Biotechnol 181(2):784–800. https://doi.org/10.1007/s12010-016-2249-7
Kazeem MO, Shah UKM, Baharuddin AS, AbdulRahman NA (2017) Prospecting agro-waste cocktail: supplementation for cellulase production by a newly isolated thermophilic B-licheniformis 2D55. Appl Biochem Biotechnol 182(4):1318–1340. https://doi.org/10.1007/s12010-017-2401-z
Elleuche S, Schroder C, Sahm K, Antranikian G (2014) Extremozymes-biocatalysts with unique properties from extremophilic microorganisms. Curr Opin Biotechnol 29:116–123. https://doi.org/10.1016/j.copbio.2014.04.003
Guerriero G, Hausman J-F, Strauss J, Ertan H, Siddiqui KS (2015) Destructuring plant biomass: focus on fungal and extremophilic cell wall hydrolases. Plant Sci 234:180–193. https://doi.org/10.1016/j.plantsci.2015.02.010
Kallioinen A, Puranen T, Siika-Aho M (2014) Mixtures of thermostable enzymes show high performance in biomass saccharification. Appl Biochem Biotechnol 173(5):1038–1056. https://doi.org/10.1007/s12010-014-0893-3
Szijarto N, Horan E, Zhang J, Puranen T, Siika-aho M, Viikari L (2011) Thermostable endoglucanases in the liquefaction of hydrothermally pretreated wheat straw. Biotechnol Biofuels 4:2
Viikari L, Vehmaanpera J, Koivula A (2012) Lignocellulosic ethanol: from science to industry. Biomass Bioenerg 46:13–24. https://doi.org/10.1016/j.biombioe.2012.05.008
Miller PS, Blum PH (2010) Extremophile-inspired strategies for enzymatic biomass saccharification. Environ Technol 31(8–9):1005–1015. https://doi.org/10.1080/09593330903536113
Bai Y, Wang J, Zhang Z, Shi P, Luo H, Huang H, Luo C, Yao B (2010) A novel family 9 beta-1,3(4)-glucanase from thermoacidophilic Alicyclobacillus sp A4 with potential applications in the brewing industry. Appl Microbiol Biotechnol 87(1):251–259. https://doi.org/10.1007/s00253-010-2452-3
Wang J, Niu C, Liu X, Chen X, Li Q (2014) Characterization of a new 1,3–1,4-β-glucanase gene from Bacillus tequilensis CGX5-1. Appl Biochem Biotechnol 173(3):826–837. https://doi.org/10.1007/s12010-014-0900-8
Grogan D, Palm P, Zillig W (1990) Isolate-B12, which harbors a virus-like element, represents a new species of the archaebacterial genus Sulfolobus, Sulfolobus shibatae, sp-nov. Arch Microbiol 154(6):594–599. https://doi.org/10.1007/BF00248842
Sambrook J, Russell DW (2001) Molecular cloning. A laboratory manual, 3 edn. Cold Spring Harbor Laboratory Press, New York
Bradford MM (1976) Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem 72(1–2):248–254. https://doi.org/10.1006/abio.1976.9999
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685. https://doi.org/10.1038/227680a0
Boyce A, Walsh G (2015) Characterisation of a novel thermostable endoglucanase from Alicyclobacillus vulcanalis of potential application in bioethanol production. Appl Microbiol Biotechnol 99(18):7515–7525. https://doi.org/10.1007/s00253-015-6474-8
Miller GL (1959) Use of dinitrosalicyclic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428. https://doi.org/10.1021/ac60147a030
Huang YW, Krauss G, Cottaz S, Driguez H, Lipps G (2005) A highly acid-stable and thermostable endo-beta-glucanase from the thermoacidophilic archaeon Sulfoloblus solfataricus. Biochem J 385:581–588. https://doi.org/10.1042/bj20041388
Girfoglio M, Rossi M, Cannio R (2012) Cellulose degradation by Sulfolobus solfataricus requires a cell-anchored endo-beta-1-4-glucanase. J Bacteriol 194(18):5091–5100. https://doi.org/10.1128/jb.00672-12
Klose H, Roeder J, Girfoglio M, Fischer R, Commandeur U (2012) Hyperthermophilic endoglucanase for in planta lignocellulose conversion. Biotechnol Biofuels. https://doi.org/10.1186/1754-6834-5-63
Maurelli L, Giovane A, Esposito A, Moracci M, Fiume I, Rossi M, Morana A (2008) Evidence that the xylanase activity from Sulfolobus solfataricus O alpha is encoded by the endoglucanase precursor gene (sso1354) and characterization of the associated cellulase activity. Extremophiles 12(5):689–700. https://doi.org/10.1007/s00792-008-0175-5
Zeldes BM, Keller MW, Loder AJ, Straub CT, Adams MWW, Kelly RM (2015) Extremely thermophilic microorganisms as metabolic engineering platforms for production of fuels and industrial chemicals. Front Microbiol. https://doi.org/10.3389/fmicb.2015.01209
Skovgaard PA, Jorgensen H (2013) Influence of high temperature and ethanol on thermostable lignocellulolytic enzymes. J Ind Microbiol Biotechnol 40(5):447–456. https://doi.org/10.1007/s10295-013-1248-8
Eckert K, Zielinski F, Lo Leggio L, Schneider E (2002) Gene cloning, sequencing, and characterization of a family 9 endoglucanase (CeIA) with an unusual pattern of activity from the thermoacidophile Alicyclobacillus acidocaldarius ATCC27009. Appl Microbiol Biotechnol 60(4):428–436. https://doi.org/10.1007/s00253-002-1131-4
Eckert K, Schneider E (2003) A thermoacidophilic endoglucanase (CelB) from Alicyclobacillus acidocaldarius displays high sequence similarity to arabinofuranosidases belonging to family 51 of glycoside hydrolases. Eur J Biochem 270(17):3593–3602. https://doi.org/10.1046/j.1432-1033.2003.03744.x
Huang XP, Monk C (2004) Purification and characterization of a cellulase (CMCase) from a newly isolated thermophilic aerobic bacterium Caldibacillus cellulovorans gen. nov., sp nov. World J Microbiol Biotechnol 20(1):85–92. https://doi.org/10.1023/B:WIBI.0000013316.12730.e7
Wang J, Gao G, Li Y, Yang L, Liang Y, ** H, Han W, Feng Y, Zhang Z (2015) Cloning, Expression, and characterization of a thermophilic endoglucanase, AcCel12B from Acidothermus cellulolyticus 11B. Int J Mol Sci 16(10):25080–25095. https://doi.org/10.3390/ijms161025080
Wang K, Luo H, Bai Y, Shi P, Huang H, Xue X, Yao B (2014) A thermophilic endo-1,4-beta-glucanase from Talaromyces emersonii CBS394.64 with broad substrate specificity and great application potentials. Appl Microbiol Biotechnol 98(16):7051–7060. https://doi.org/10.1007/s00253-014-5680-0
Hua C, Li W, Han W, Wang Q, Bi P, Han C, Zhu L (2018) Characterization of a novel thermostable GH7 endoglucanase from Chaetomium thermophilum capable of xylan hydrolysis. Int J Biol Macromol 117:342–349. https://doi.org/10.1016/j.ijbiomac.2018.05.189
Ando S, Ishida H, Kosugi Y, Ishikawa K (2002) Hyperthermostable endoglucanase from Pyrococcus horikoshii. Appl Environ Microbiol 68(1):430–433. https://doi.org/10.1128/AEM.68.1.430-433.2002
Bauer MW, Driskill LE, Callen W, Snead MA, Mathur EJ, Kelly RM (1999) An endoglucanase, eg1A, from the hyperthermophilic archaeon Pyrococcus furiosus hydrolyzes β-1,4 bonds in mixed-linkage (1→3),(1→4)-β-D-glucans and cellulose. J Bacteriol 181(1):284–290
Plascencia-Espinosa M, Santiago-Hernández A, Pavón-Orozco P, Vallejo-Becerra V, Trejo-Estrada S, Sosa-Peinado A, Benitez-Cardoza CG, Hidalgo-Lara ME (2014) Effect of deglycosylation on the properties of thermophilic invertase purified from the yeast Candida guilliermondii MpIIIa. Process Biochem 49(9):1480–1487. https://doi.org/10.1016/j.procbio.2014.05.022
Zou S, Huang S, Kaleem I, Li C (2013) N-glycosylation enhances functional and structural stability of recombinant β-glucuronidase expressed in Pichia pastoris. J Biotechnol 164(1):75–81. https://doi.org/10.1016/j.jbiotec.2012.12.015
Han YM, Lei XG (1999) Role of glycosylation in the functional expression of an Aspergillus niger phytase (phyA) in Pichia pastoris. Arch Biochem Biophys 364(1):83–90. https://doi.org/10.1006/abbi.1999.1115
Hoiberg-Nielsen R, Fuglsang CC, Arleth L, Westh P (2006) Interrelation ships of glycosylation and aggregation kinetics for Peniophora lycii phytase. Biochemistry 45(15):5057–5066. https://doi.org/10.1021/bi0522955
Méndez Arias J, de Oliveira Moraes A, Modesto LFA, de Castro AM, Pereira N (2017) Addition of surfactants and non-hydrolytic proteins and their influence on enzymatic hydrolysis of pretreated sugarcane bagasse. Appl Biochem Biotechnol 181(2):593–603. https://doi.org/10.1007/s12010-016-2234-1
Parnthong J, Kungsanant S, Chavadej S (2018) The influence of nonionic surfactant adsorption on enzymatic hydrolysis of oil palm fruit bunch. Appl Biochem Biotechnol 1–14. https://doi.org/10.1007/s12010-018-2783-6
Rocha-Martín J, Martinez-Bernal C, Pérez-Cobas Y, Reyes-Sosa FM, García BD (2017) Additives enhancing enzymatic hydrolysis of lignocellulosic biomass. Bioresour Technol 244:48–56. https://doi.org/10.1016/j.biortech.2017.06.132
Deep K, Poddar A, Das SK (2016) Cloning, overexpression, and characterization of halostable, solvent-tolerant novel β-endoglucanase from a marine bacterium Photobacterium panuliri LBS5T (DSM 27646T). Appl Biochem Biotechnol 178(4):695–709. https://doi.org/10.1007/s12010-015-1903-9
Huang XM, Li QQ, Chen XL, Fan JX, Xu XH, Sun XD, Li DY, Zhao HX (2017) Expression and characteristics of an endoglucanase from Trichoderma atroviride (TaEGII) in Saccharomyces cerevisiae. Appl Biochem Biotechnol 182(3):1158–1170. https://doi.org/10.1007/s12010-016-2389-9
Xu X, Li J, Zhang W, Huang H, Shi P, Luo H, Liu B, Zhang Y, Zhang Z, Fan Y, Yao B (2015) A neutral thermostable beta-1,4-glucanase from Humicola insolens Y1 with potential for applications in various industries. PLoS ONE. https://doi.org/10.1371/journal.pone.0124925
Miotto LS, de Rezende CA, Bernardes A, Serpa VI, Tsang A, Polikarpov I (2014) The characterization of the endoglucanase Cel12A from Gloeophyllum trabeum reveals an enzyme highly active on beta-glucan. PLoS ONE. https://doi.org/10.1371/journal.pone.0108393
McCarthy T, Hanniffy O, Savage AV, Tuohy MG (2003) Catalytic properties and mode of action of three endo-beta-glucanases from Talaromyces emersonii on soluble beta-1,4- and beta-1,3–1,4-linked glucans. Int J Biol Macromol 33(1–3):141–148. https://doi.org/10.1016/s0141-8130(03)00080-1
Acknowledgements
This work was supported by Science Foundation Ireland/Enterprise Ireland Technology Innovation Development Award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Boyce, A., Walsh, G. Expression and characterisation of a thermophilic endo-1,4-β-glucanase from Sulfolobus shibatae of potential industrial application. Mol Biol Rep 45, 2201–2211 (2018). https://doi.org/10.1007/s11033-018-4381-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-018-4381-7