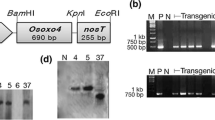
Abstract
Late blight, caused by Phytophthora infestans, is the most devastating potato disease worldwide and results in catastrophic production losses. Reactive oxygen species (ROS) are signal molecules utilized at the early stage of plant immunity. Specifically, anionic peroxidases are reported to be involved in many plants’ defense systems. However, there is limited information about the function of anionic peroxidase genes in potato immunity. Here, we report that StPOPA, a gene encoding a suberization-associated anionic peroxidase, was induced by P. infestans infection, mechanical damage, as well as jasmonic acid and ethylene treatments. Overexpression of StPOPA gene in potato enhanced potato resistance against P. infestans by promoting the accumulation of callose in the cell wall and ROS in the cytoplasm, and then the callose and ROS restricted the infection and spreading of the disease possibly by purposeful programmed cell death. Our results suggest that StPOPA plays a positive role in potato resistance to immunochallenge, and this gene could be used for the genetic improvement of resistance against potato late blight.





Similar content being viewed by others
References
Almagro L, Gómez Ros LV, Belchinavarro S, Bru R, Ros BA, Pedreño MA (2009) Class III peroxidases in plant defence reactions. J Exp Bot 60:377–390
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Bernards MA, Fleming WD, Llewellyn DB, Priefer R, Yang X, Sabatino A, Plourde GL (1999) Biochemical characterization of the suberization-associated anionic peroxidase of potato. Plant Physiol 121:135–145
Boller T, He SY (2009) Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science 324:742–744
Burel C, Berthe T, Mery JC, Morvan C, Balange AP (1994) Isoelectric focusing analysis of peroxidases in flax seedling hypocotyls grown in different light conditions. Plant Physiol Biochem 32:853–860
Chen N et al (2018) Molecular marker development and primary physical map construction for the tuber shape Ro gene locus in diploid potato (Solanum tuberosum L.). Mol Breed 39:6
Dangl JL, Horvath DM, Staskawicz BJ (2013) Pivoting the plant immune system from dissection to deployment. Science 341:746–751
Daudi A, Cheng Z, O’Brien JA, Mammarella N, Khan S, Ausubel FM, Bolwell GP (2012) The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell 24:275–287
Delledonne M, Zeier J, Marocco A, Lamb C (2001) Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci U S A 98:13454–13459
Doke N (1983) Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components. Physiol Plant Pathol 23:345–357
Du J, Tian Z, Liu J, Vleeshouwers VG, Shi X, **e C (2013) Functional analysis of potato genes involved in quantitative resistance to Phytophthora infestans. Mol Biol Rep 40:957–967
Foyer CH, Noctor G (2000) Oxygen processing in photosynthesis: regulation and signalling. New Phytol 146:359–388
Frederickson Matika DE, Loake GJ (2013) Redox regulation in plant immune function. Antioxid Redox Signal 21:1373–1388
Fukuoka S, Saka N, Koga H, Ono K, Shimizu T, Ebana K, Hayashi N, Takahashi A, Hirochika H, Okuno K, Yano M (2009) Loss of function of a proline-containing protein confers durable disease resistance in rice. Science 325:998–1001
Giraldo MC, Valent B (2013) Filamentous plant pathogen effectors in action. Nat Rev Microbiol 11:800
Grant JJ, Loake GJ (2000) Role of reactive oxygen intermediates and cognate redox signaling in disease resistance. Plant Physiol 124:21–29
He Q, Mclellan H, Boevink PC, Sadanandom A, **e C, Birch PRJ, Tian Z (2015) U-box E3 ubiquitin ligase PUB17 acts in the nucleus to promote specific immune pathways triggered by Phytophthora infestans. J Exp Bot 66:3189–3199
Hemavathi et al (2011) Biochemical analysis of enhanced tolerance in transgenic potato plants overexpressing d-galacturonicacidreductase gene in response to various abiotic stresses. Mol Breed 28:105–115
Inupakutika MA, Sengupta S, Devireddy AR, Azad RK, Mittler R (2016) The evolution of reactive oxygen species metabolism. J Exp Bot 67:5933–5943
Jiang R, Li J, Tian Z, du J, Armstrong M, Baker K, Tze-Yin Lim J, Vossen JH, He H, Portal L, Zhou J, Bonierbale M, Hein I, Lindqvist-Kreuze H, **e C (2018) Potato late blight field resistance from QTL dPI09c is conferred by the NB-LRR gene R8. J Exp Bot 69:1545–1555
Jones JDG, Dangl JL (2006) The plant immune system. Nature 444:323–329
Jouili H, Bouazizi H, Ferjani EE (2011) Plant peroxidases: biomarkers of metallic stress. Acta Physiol Plant 33:2075–2082
Jwa N-S, Hwang BK (2017) Convergent evolution of pathogen effectors toward reactive oxygen species signaling networks in plants. Front Plant Sci 8:1687
Kadota Y, Sklenar J, Derbyshire P, Stransfeld L, Asai S, Ntoukakis V, Jones JD, Shirasu K, Menke F, Jones A, Zipfel C (2014) Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol Cell 54:43–55
Klotz KL, Liu TTY, Liu L, Lagrimini LM (1998) Expression of the tobacco anionic peroxidase gene is tissue-specific and developmentally regulated. Plant Mol Biol 36:509–520
Lagrimini LM, Joly RJ, Dunlap JR, Liu TT (1997) The consequence of peroxidase overexpression in transgenic plants on root growth and development. Plant Mol Biol 33:887–895
Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 48:251–275
Lehmann S, Serrano M, L'Haridon F, Tjamos SE, Metraux JP (2015) Reactive oxygen species and plant resistance to fungal pathogens. Phytochemistry 112:54–62
Leon JC, Alpeeva IS, Chubar TA, Galaev IY, Csoregi E, Sakharov IY (2002) Purification and substrate specificity of peroxidase from sweet potato tubers. Plant Sci 163:1011–1019
Li Y, Tian Z, Liu J, **e C (2009) Comparative cDNA-AFLP analysis reveals that DL-beta-amino-butyric acid induces resistance through early activation of the host-defense genes in potato. Physiol Plantarum 136:19–29
Li W et al (2017) A natural allele of a transcription factor in rice confers broad-spectrum blast resistance. Cell 170:114–126
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408
Milla MAR, Maurer A, Huete AR, Gustafson JP (2010) Glutathione peroxidase genes in Arabidopsis are ubiquitous and regulated by abiotic stresses through diverse signaling pathways. Plant J 36:602–615
Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, van Breusegem F (2011) ROS signaling: the new wave? Trends Plant Sci 16:300–309
Mohan R, Kolattukudy PE (1990) Differential activation of expression of a suberization-associated anionic peroxidase gene in near-isogenic resistant and susceptible tomato lines by elicitors of Verticillium albo-atrum. Plant Physiol 92:276–280
Montillet JL, Nicole M (2000) Salicylic acid mediated by the oxidative burst is a key molecule in local and systemic responses of cotton challenged by an avirulent race of Xanthomonas campestris pv malvacearum. Plant Physiol 122:757–766
Navrot N, Collin V, Gualberto J, Gelhaye E, Hirasawa M, Rey P, Knaff DB, Issakidis E, Jacquot JP, Rouhier N (2006) Plant glutathione peroxidases are functional peroxiredoxins distributed in several subcellular compartments and regulated during biotic and abiotic stresses. Plant Physiol 142:1364–1379
Nürnberger T, Brunner F, Kemmerling B, Piater L (2004) Innate immunity in plants and animals: striking similarities and obvious differences. Immunol Rev 198:249–266
Oliveira HP, Silva RGG, Oliveira JTA, Sousa DOB, Pereira ML, Souza PFN, Soares AA, Gomes VM, Monteiro-Moreira ACO, Moreno FBMB, Vasconcelos IM (2017) A novel peroxidase purified from Marsdenia megalantha latex inhibits phytopathogenic fungi mediated by cell membrane permeabilization. Int J Biol Macromol 96:743–753
Pandey VP, Awasthi M, Singh S, Tiwari S, Dwivedi UN (2017) A comprehensive review on function and application of plant peroxidases. Anal Biochem 6(01) DOI: https://doi.org/10.4172/2161-1009.1000308
Passardi F, Cosio C, Penel C, Dunand C (2005) Peroxidases have more functions than a Swiss army knife. Plant Cell Rep 24:255–265
Quiroga M, Guerrero C, Botella MA, Barceló A, Amaya I, Medina MI, Alonso FJ, de Forchetti SM, Tigier H, Valpuesta V (2000) A tomato peroxidase involved in the synthesis of lignin and suberin. Plant Physiol 122:1119–1127
Reumann S, Bartel B (2016) Plant peroxisomes: recent discoveries in functional complexity, organelle homeostasis, and morphological dynamics. Curr Opin Plant Biol 34:17–26
Roberts E, Kutchan T, Kolattukudy PE (1988) Cloning and sequencing of cDNA for a highly anionic peroxidase from potato and the induction of its mRNA in suberizing potato tubers and tomato fruits. Plant Mol Biol 11:15–26
Sorokan AV, Kuluev BR, Burkhanova GF, Maksimov IV (2014) RNA silencing of the anionic peroxidase gene impairs potato plant resistance to Phytophthora infestans (Mont.) de Bary. Mol Biol 48:709–717
Tian Z, He Q, Wang H, Liu Y, Zhang Y, Shao F, **e C (2015) The potato ERF transcription factor StERF3 negatively regulates resistance to Phytophthora infestans and salt tolerance in potato. Plant Cell Physiol 56:992–1005
Torres MA (2010) ROS in biotic interactions. Physiol Plantarum 138:414–429
Torres MA, Jones JD, Dangl JL (2006) Reactive oxygen species signaling in response to pathogens. Plant Physiol 141:373–378
Valério L, Meyer MD, Penel C, Dunand C (2004) Expression analysis of the Arabidopsis peroxidase multigenic family. Phytochemistry 65:1331–1342
Voinnet O, Rivas S, Mestre P, Baulcombe D (2010) An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J 33:949–956
Welinder KG (1992) Superfamily of plant, fungal and bacterial peroxidases. Curr Opin Struct Biol 2:388–393
Xue RF et al (2014) Cloning and characterization of a novel secretory root-expressed peroxidase gene from common bean (Phaseolus vulgaris L.) infected with Fusarium oxysporum f. sp. Phaseoli. Mol Breeding 34:855–870
Funding
This research was supported by grants from the National Natural Science Foundation of China (31261140362).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(XLS 27 kb)
Rights and permissions
About this article
Cite this article
Yang, Y., Jiang, R., Wang, H. et al. StPOPA, encoding an anionic peroxidase, enhances potato resistance against Phytophthora infestans. Mol Breeding 40, 16 (2020). https://doi.org/10.1007/s11032-019-1093-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-019-1093-1