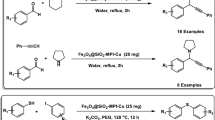
Abstract
Three-component reaction of aldehydes with 3-(1H-indol-3-yl)-3-oxopropanenitrile and 1H-1,2,4-triazol-5-amine under the solvent-free condition at 70 °C was effectively performed in the presence of 2 mg of polyionic magnetic nanoparticles with pyrazine bridge [Fe3O4@SiO2@(CH2)3]2-Pyrazinium-[TCM]2 as a catalyst for the synthesis of 7-aryl-5-(1H-indol-3-yl)-[1,2,4]triazolo[1,5-a]pyrimidine-6-carbonitriles via a cooperative anomeric-based oxidation. The polyionic magnetic nanoparticles catalyst was simply recovered and reused four successive runs. The morphology and structure of MNPs catalyst were investigated by numerous techniques such as XRD, FT-IR, EDX, WDX, FE-SEM, TEM, TGA, DTA, and VSM. The obtained products are reported for the first time that were identified by various analyses techniques such as melting point, FT-IR, 1H NMR, 13C NMR, and elemental analysis (CHN). A term entitled a cooperative geminal-vinylogous anomeric-based oxidation was introduced for the latter step of the reaction mechanism for the first time.
Graphic abstract

Synthesis of 7-aryl-5-(1H-indol-3-yl)-[1,2,4]triazolo[1,5-a]pyrimidine-6-carbonitriles by using [Fe3O4@SiO2@(CH2)3]2-Pyrazinium-[TCM]2 MNPs as a catalyst.
















Similar content being viewed by others
References
Lucarelli C, Vaccari A (2011) Examples of heterogeneous catalytic processes for fine chemistry. Green Chem 13:1941–1949. https://doi.org/10.1039/C0GC00760A
Niknam K, Hashemi H, Karimzadeh M, Saberi D (2020) Recent advances in preparation and application of sulfonic acid derivatives bonded to inorganic supports. J Iran Chem Soc 17:3095–3178. https://doi.org/10.1007/s13738-020-01997-w
Lanzafame P, Perathoner S, Centi G, Gross S, Hensen EJM (2017) Grand challenges for catalysis in the science and technology roadmap on catalysis for Europe: moving ahead for a sustainable future. Catal Sci Technol 7:5182–5194. https://doi.org/10.1039/C7CY01067B
Gawande MB, Brancoa PS, Varma RS (2013) Nano-magnetite (Fe3O4) as a support for recyclable catalysts in the development of sustainable methodologies. Chem Soc Rev 42:3371–3393. https://doi.org/10.1039/C3CS35480F
Balanta A, Godard C, Claver C (2011) Pdnanoparticles for C–C coupling reactions. Chem Soc Rev 40:4973–4985. https://doi.org/10.1039/C1CS15195A
Burda C, Chen X, Narayanan R, El-Sayed MA (2005) Chemistry and properties of nanocrystals of different shapes. Chem Rev 105:1025–1102. https://doi.org/10.1021/cr030063a
Yin L, Liebscher J (2007) Carbon−carbon coupling reactions catalyzed by heterogeneous palladium catalysts. Chem Rev 107:133–173. https://doi.org/10.1021/cr0505674
Shylesh S, Schnemann V, Thiel WR (2010) Magnetisch abtrennbare nanokatalysatoren: Brücken zwischen homogener und heterogener katalyse. Angew Chem 122:3504–3537. https://doi.org/10.1002/ange.200905684
Astruc D, Boisselier E, Ornelas C (2010) Dendrimers designed for functions: from physical, photophysical, and supramolecular properties to applications in sensing, catalysis, molecular electronics, photonics, and nanomedicine. Chem Rev 110:1857–1959. https://doi.org/10.1021/cr900327d
Davarpanah J, Kiasat AR (2013) Catalytic application of silver nanoparticles immobilized to rice husk-SiO2-aminopropylsilane composite as recyclable catalyst in the aqueous reduction of nitroarenes. Catal Commun 41:6–11. https://doi.org/10.1016/j.catcom.2013.06.020
Zolfiol MA, Khakyzadeh V, Moosavi-Zare AR, Rostami A, Zare A, Iranpoor N, Beyzavi MH, Luque R (2013) A highly stable and active magnetically separable Pd nanocatalyst in aqueous phase heterogeneously catalyzed couplings. Green Chem 15:2132–2140. https://doi.org/10.1039/C3GC40421H
Nikoofar K, Ekhtiari NS (2021) Green synthesis of some 3-(α, α-diarylmethyl)indoles by bio-nanocomposite from embedding L–histidinium trichloroacetate ionic liquid on functionalized magnetite (L–His+CCl3CO2−@PEG@SiO2–nano Fe3O4). Mol Diversity. https://doi.org/10.1007/s11030-021-10268-6
Shahabi Nejad M, Seyedi N, Sheibani H, Behzadi S (2019) Synthesis and characterization of Ni (II) complex functionalized silica-based magnetic nanocatalyst and its application in C–N and C–C cross-coupling reactions. Mol Divers 23:527–539. https://doi.org/10.1007/s11030-018-9888-2
Soleimani E, Torkaman S, Sepahvand H, Ghorbani S (2019) Ciprofloxacin-functionalized magnetic silica nanoparticles: as a reusable catalyst for the synthesis of 1H-chromeno[2,3-d]pyrimidine-5-carboxamides and imidazo[1,2-a]pyridines. Mol Divers 23:739–749. https://doi.org/10.1007/s11030-018-9907-3
Shahamat Z, Nemati F, Elhampour A (2020) One-pot synthesis of propargylamines using magnetic mesoporous polymelamine formaldehyde/zinc oxide nanocomposite as highly efficient, eco-friendly and durable nanocatalyst: optimization by DOE approach. Mol Divers 24:691–706. https://doi.org/10.1007/s11030-019-09977-w
Phillips MA, Gujjar R, Malmquist NA, White J, El-Mazouni F, Baldwin J, Rathod PK (2008) Triazolopyrimidine-based dihydroorotate dehydrogenase inhibitors with potent and selective activity against the malaria parasite plasmodium falciparum. J Med Chem 51:3649–3653. https://doi.org/10.1021/jm8001026
Gujjar R, Marwaha A, El-Mazouni F, White J, White KL, Creason S, Shackleford DM, Baldwin J, Charman WN, Buckner FS, Charman S, Rathod PK, Phillips MA (2009) Identification of a metabolically stable triazolopyrimidine-based dihydroorotate dehydrogenase inhibitor with antimalarial activity in mice. J Med Chem 52:1864–1872. https://doi.org/10.1021/jm801343r
Bera H, Chigurupati S (2016) Recent discovery of non-nucleobase thymidine phosphorylase inhibitors targeting cancer. Eur J Med Chem 124:992–1003. https://doi.org/10.1016/j.ejmech.2016.10.032
Pracharova J, Saltarella T, Muchova TR, Novakova O, Scintilla S, Novohradsky V, Novakova O, Intini FP, Pacifico C, Natile G, Ilik P, Brabec V, Kasparkova J (2015) Novel antitumor cisplatin and transplatin derivatives containing 1-methyl-7-azaindole: synthesis, characterization, and cellular responses. J Med Chem 58:847–859. https://doi.org/10.1021/jm501420k
Caballero AB, Rodrıguez-Dieguez A, Lezama L, Barea E, Salas JM (2011) Structural and magnetic properties of three novel complexes with the versatile ligand 5-methyl-1,2,4-triazolo [1,5-a] pyrimidin-7(4H)-one. Dalton Trans 40:5180–5187. https://doi.org/10.1039/C0DT01416H
Zhang N, Ayral-Kaloustian S, Nguyen T, Afragola J, Hernandez R, Lucas J, Gibbons J, Beyer C (2007) Synthesis and SAR of [1,2,4]triazolo[1,5-a]pyrimidines, a class of anticancer agents with a unique mechanism of tubulin inhibition. J Med Chem 50:319–327. https://doi.org/10.1021/jm060717i
Suresh L, Kumar PSV, Vinodkumar T, Chandramouli GVP (2016) Heterogeneous recyclable nano-CeO2 catalyst: efficient and eco-friendly synthesis of novel fused triazolo and tetrazolo pyrimidine derivatives in aqueous medium. RSC Adv 6:68788–68797. https://doi.org/10.1039/C6RA16307F
Radwan MAA, Alminderej FM, Awad HM (2020) One-pot multicomponent synthesis and cytotoxic evaluation of novel 7-substituted-5-(1H-indol-3-yl)tetrazolo[1,5-a]pyrimidine-6-carbonitrile. Molecules 25:255–255. https://doi.org/10.3390/molecules25020255
Radwan MAA, Alminderej FM, Tolan HEM, Awad HM (2020) One-pot three-component synthesis of new triazolopyrimidine derivatives bearing indole moiety as antiproliferative agents. J Appl Pharm Sci 10:12–22. https://doi.org/10.7324/JAPS.2020.10902
El-Gendy MMA, Shaaban M, Shaaban KA, El-Bondkly AM, Laatsch H (2008) Essramycin: a first triazolopyrimidine antibiotic isolated from nature. J Antibiot 61:149–157. https://doi.org/10.1038/ja.2008.124
Alabugin IV (2016) Stereoelectronic effects: the bridge between structure and reactivity. Wiley, UK
Curran DP, Suh YG (1987) Selective mono-Claisen rearrangement of carbohydrate glycals. a chemical consequence of the vinylogous anomeric effect. Carbohydr Res 17:161–191. https://doi.org/10.1016/S0008-6215(00)90885-1
Denmark SE, Dappen MS, Sear NL, Jacobs RT (1990) The vinylogous anomeric effect in 3-alkyl-2-chlorocyclohexanone oximes and oxime ethers. J Am Chem Soc 112:3466–3474. https://doi.org/10.1021/ja00165a034
Jakel C, Dotz KH (2001) Organotransition metal modified sugars: Part 22. Direct metalation of glycals: short and efficient routes to diversely protected stannylated glycals. J Organomet Chem 624:172–185. https://doi.org/10.1016/S0022-328X(00)00920-7
Drew MD, Wall MC, Kim JT (2012) Stereoselective propargylation of glycals with allenyltributyltin (IV) via a Ferrier type reaction. Tetrahedron Lett 53:2833–2836. https://doi.org/10.1016/j.tetlet.2012.03.115
Nowacki A, Walczak D, Liberek B (2012) Fully acetylated 1,5-anhydro-2-deoxypent-1-enitols and 1,5-anhydro-2,6-dideoxyhex-1-enitols in DFT level theory conformational studies. Carbohydr Res 352:177–185. https://doi.org/10.1016/j.carres.2012.02.008
Nowacki A, Liberek B (2013) Acetylated methyl 1,2-dideoxyhex-1-enopyranuronates in density functional theory conformational studies. Carbohydr Res 371:1–7. https://doi.org/10.1016/j.carres.2013.01.009
Asgari M, Nori-Shargh D (2017) Exploring the impacts of the vinylogous anomeric effect on the synchronous early and late transition states of the hydrogen molecule elimination reactions of cis-3,6-dihalocyclohexa-1,4-dienes. Struct Chem 28:1803–1814. https://doi.org/10.1007/s11224-017-0959-2
Nowacki A, Liberek B (2018) Comparative conformational studies of 3,4,6-tri-O-acetyl-1,5-anhydro-2-deoxyhex-1-enitols at the DFT level. Carbohydr Res 462:13–27. https://doi.org/10.1016/j.carres.2018.03.013
Ferrier RJ, Sankey GH (1966) Unsaturated carbohydrates. Part VI. a modified synthesis of 2-hydroxyglycal esters, and their conversion into esters of 2,3-didehydro-3-deoxyaldoses. J Chem Soc C. https://doi.org/10.1039/J39660002345
Yarie M (2017) Catalytic anomeric based oxidation. Iran J Catal 7:85–88
Yarie M (2020) Catalytic vinylogous anomeric based oxidation (Part I). Iran J Catal 10:79–83
Zolfigol MA, Ayazi-Nasrabadi R, Baghery S, Khakyzadeh V, Azizian S (2016) Applications of a novel nano magnetic catalyst in the synthesis of 1,8-dioxo-octahydroxanthene and dihydropyrano [2,3-c] pyrazole derivatives. J Mol Catal A Chem 418:54–67. https://doi.org/10.1016/j.molcata.2016.03.027
Zolfigol MA, Ayazi-Nasrabadi R, Baghery S (2016) The first urea-based ionic liquid-stabilized magnetic nanoparticles: an efficient catalyst for the synthesis of bis(indolyl)methanes and pyrano[2,3-d]pyrimidinone derivatives. Appl Organometal Chem 30:273–281. https://doi.org/10.1002/aoc.3428
Trofimenko S, Little EL, Mower HF (1962) Tricyanomethane (cyanoform), carbamyldicyanomethane, and their derivatives. J Org Chem 27:433–438. https://doi.org/10.1021/jo01049a021
Carboni RA (1959) Tetracyanoethylene: Ethenetetracarbonitrile. Org Synth 39:64–64. https://doi.org/10.1002/0471264180.os039.23
Beaumont RC, Aspin KB, Demas TJ, Hoggatt JH, Potter GE (1984) Oxidation of the tricyanomethanide ion: the tricyanocarbonium ion. Inorg Chem Acta 84:141–147. https://doi.org/10.1016/S0020-1693(00)82399-3
Banert K, Hagedorn M (2019) Tricyanomethane and its salts with nitrogen bases: a correction of sixteen reports. Synlett 30:1427–1430. https://doi.org/10.1055/s-0037-1611846
Banert K, Chityala M, Hagedorn M, Beckers H, Stgker T, Riedel S, Rgffer T, Lang H (2017) Tricyanomethane and its ketenimine tautomer: generation from different precursors and analysis in solution, argon matrix, and as a single crystal. Angew Chem Int Ed 56:9582–9586. https://doi.org/10.1002/anie.201704561
Ghasemi P, Yarie M, Zolfigol MA, Taherpour A (2020) Ionically tagged magnetic nanoparticles with urea linkers: application for preparation of 2-aryl-quinoline-4-carboxylic acids via an anomeric-based oxidation mechanism. ACS Omega 5:3207–3217. https://doi.org/10.1021/acsomega.9b03277
Dashteh M, Zolfigol MA, Khazaei A, Baghery S, Yarie M, Makhdoomi S, Safaiee M (2020) Synthesis of cobalt tetra-2,3-pyridiniumporphyrazinato with sulfonic acid tags as an efficient catalyst and its application for the synthesis of bicyclic ortho-aminocarbonitriles, cyclohexa-1,3-dienamines and 2-amino-3-cyanopyridines. RSC Adv 10:27824–27834. https://doi.org/10.1039/D0RA02172E
Zolfigol MA, Baghery S, Moosavi-Zare AR, Vahdat SM, Alinezhad H, Norouzi M (2015) Design of 1-methylimidazolium tricyanomethanide as the first nanostructured molten salt and its catalytic application in the condensation reaction of various aromatic aldehydes, amides and β-naphthol compared with tin dioxide nanoparticles. RSC Adv 5:45027–45037. https://doi.org/10.1039/C5RA02718G
Wan Y, Yuan R, Zhang FR, Pang LL, Ma R, Yue CH, Lin W, Yin W, Bo R, Wu H (2011) One-pot synthesis of N2-substituted 2-amino-4-aryl-5,6,7,8-tetrahydroquinoline-3-carbonitrile in basic ionic liquid [bmim]OH. Synth Commun 41:2997–3015. https://doi.org/10.1080/00397911.2010.516459
Zolfigol M, Gholami H, Khakyzadeh V (2014) Principles of organic synthesis with a new approach, 3rd edn. Bu-Ali Sina University Publishers, Hamedan, Iran, p 26
Erhardt JM, Wuest JD (1980) Transfer of hydrogen from orthoamides. Reduction of protons to molecular hydrogen. J Am Chem Soc 102:6363–6364. https://doi.org/10.1021/ja00540a043
Atkins TJ (1980) Tricyclic trisaminomethanes. J Am Chem Soc 102:6364–6365. https://doi.org/10.1021/ja00540a044
Erhardt JM, Grover ER, Wuest JD (1980) Transfer of hydrogen from orthoamides. Synthesis, structure, and reactions of hexahydro-6bH-2a,4a,6a-triazacyclopenta [cd] pentalene and perhydro-3a,6a,9a-triazaphenalene. J Am Chem Soc 102:6365–6369. https://doi.org/10.1021/ja00540a045
Zolfigol MA, Afsharnadery F, Baghery S, Salehzadeh S, Maleki F (2015) Catalytic applications of {[HMIM]C(NO2)3}: as a nano ionic liquid for the synthesis of pyrazole derivatives under green conditions and a mechanistic investigation with a new approach. RSC Adv 5:75555–75568. https://doi.org/10.1039/C5RA16289K
Moosavi-Zare AR, Zolfigol MA, Rezanejad Z (2016) Trityl chloride promoted the synthesis of 3-(2,6-diarylpyridin-4-yl)-1H-indoles and 2,4,6-triarylpyridines by in situ generation of trityl carbocation and anomeric based oxidation in neutral media. Can J Chem 94:626–630. https://doi.org/10.1139/cjc-2015-0629
Zolfigol MA, Kiafar M, Yarie M, Taherpour A, Fellowes T, Hancok AN, Yari A (2017) A convenient method for preparation of 2-amino-4,6-diphenylnicotinonitrile using HBF4 as an efficient catalyst via an anomeric based oxidation: a joint experimental and theoretical study. J Mol Struct 1137:674–680. https://doi.org/10.1016/j.molstruc.2017.02.083
Baghery S, Zolfigol MA, Maleki F (2017) [TEATNM] and [TEATCM] as novel catalysts for the synthesis of pyridine-3, 5-dicarbonitriles via anomeric-based oxidation. New J Chem 41:9276–9290. https://doi.org/10.1039/C7NJ01934C
Bodaghifard M, Shafi S (2021) Ionic liquid-immobilized hybrid nanomaterial: an efficient catalyst in the synthesis of benzimidazoles and benzothiazoles via anomeric-based oxidation. J Iran Chem Soc 18:677–687. https://doi.org/10.1007/s13738-020-02055-1
Alabugin IV, Gomes GDP, Abdo MA (2019) Hyperconjugation. WIREs Comput Mol Sci 9:e1389. https://doi.org/10.1002/wcms.1389
Alabugin IV, Gilmore KM, Peterson PW (2011) Hyperconjugation. WIREs Comput Mol Sci 1:109–141. https://doi.org/10.1002/wcms.6
Greenway KT, Bischoff AG, Pinto BM (2012) Probing hyperconjugation experimentally with the conformational deuterium isotope effect. J Org Chem 77:9221–9226. https://doi.org/10.1021/jo3017988
Mo Y (2010) Computational evidence that hyperconjugative interactions are not responsible for the anomeric effect. Nature Chem 2:666–671. https://doi.org/10.1038/nchem.721
Li W, Tian S, Wu L (2013) Regioselective multi-component synthesis of 7-aryl-benzo[h][1,2,4]-triazolo[5,1-b]quinazoline-5,6-diones catalyzed by n-propylsulfonated γ-Al2O2. Bull Korean Chem Soc 34:2825–2828. https://doi.org/10.5012/bkcs.2013.34.9.2825
Wu L, Zhang C, Li W (2013) Regioselective synthesis of 6-aryl-benzo[h][1,2,4]-triazolo[5,1-b]quinazoline-7,8-diones as potent antitumoral agents. Bioorg Med Chem Lett 23:5002–5005. https://doi.org/10.1016/j.bmcl.2013.06.040
Sompalle R, Roopan SM, Al-Dhabi NA, Suthindhiran K, Sarkar G, Arasu MV (2016) 1,2,4-Triazolo-quinazoline-thiones: non-conventional synthetic approach, study of solvatochromism and antioxidant assessment. J Photochem Photobiol B Biol 162:232–239. https://doi.org/10.1016/j.jphotobiol.2016.06.051
Kidwai M, Chauhan R (2013) Nafion-H® catalyzed efficient one-pot synthesis of triazolo[5,1-b]quinazolinones and triazolo[1,5-a]pyrimidines: a green strategy. J Mol Catal A Chem 377:1–6. https://doi.org/10.1016/j.molcata.2013.04.014
Acknowledgements
We thank Bu-Ali Sina University, Iran National Science Foundation (INSF) (Grant Number: 98001912), for financial support to our research group.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing for financial interests or personal relationships that could have seemed to influence the work described in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Appendix A. Supplemental File
Appendix A. Supplemental File
The Supplemental File affords materials and methods, CHNS data, and spectral images of FT-IR, 1H NMR and 13C NMR of title products
Rights and permissions
About this article
Cite this article
Dashteh, M., Baghery, S., Zolfigol, M.A. et al. Application of polyionic magnetic nanoparticles as a catalyst for the synthesis of carbonitriles with both indole and triazole moieties via a cooperative geminal-vinylogous anomeric-based oxidation. Mol Divers 26, 2407–2426 (2022). https://doi.org/10.1007/s11030-021-10339-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-021-10339-8