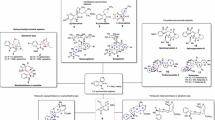
Abstract
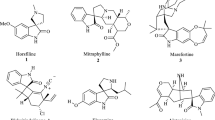
Spirocyclic compounds fascinate the synthetic chemists due to their privileged ring system and efficacy in drug discovery. Many natural compounds comprise spirocyclic moiety in their skeleton and are effective in pharmaceutical industry. Over the years, many synthetic methodologies have been established for the construction of spirocyclic compounds. In this review, recent synthetic approaches to accessing various spirocompounds comprising six-membered carbocycles/heterocycles have been summarized.
Graphic abstract





























































































Similar content being viewed by others
References
Moss GP (1999) Extension and revision of the nomenclature for spiro compounds. Pure Appl Chem 71:531–558. https://doi.org/10.1351/pac199971030531
Saraswat P, Jeyabalan G, Hassan MZ, Rahman MU, Nyola NK (2016) Review of synthesis and various biological activities of spiro heterocyclic compounds comprising oxindole and pyrrolidine moieties. Synth Commun 46:1643–1664. https://doi.org/10.1080/00397911.2016.1211704
Khanna P, Panda SS, Khanna L, Jain SC (2014) Aqua mediated synthesis of spirocyclic compounds. Min Rev Org Chem 11:73–86. https://doi.org/10.2174/1570193X1101140402101831
Chin YW, Salim AA, Su BN, Mi Q, Chai HB, Riswan S, Kardono LB, Ruskandi A, Farnsworth NR, Swanson SM, Kinghorn AD (2008) Potential anticancer activity of naturally occurring and semisynthetic derivatives of aculeatins A and B from Amomum aculeatum. J Nat Prod 71:390–395. https://doi.org/10.1021/np070584j
Litvinov YM, Mortikov VY, Shestopalov AM (2008) Versatile three-component procedure for combinatorial synthesis of 2-aminospiro [(3′H)-indol-3′,4-(4H)-pyrans]. J Comb Chem 10:741–745. https://doi.org/10.1021/cc800093q
Jursic BS, Stevens ED (2004) Preparation of nitrogen heterocycles of spiro [furo [2, 3-d]-pyrimidine] pyrimidine derivatives. Synth Commun 34:3915–3923. https://doi.org/10.1081/SCC-200034786
Jadidi K, Ghahremanzadeh R, Bazgir A (2009) Efficient synthesis of spiro [chromeno [2, 3-d] pyrimidine-5, 3′-indoline]-tetraones by a one-pot and three-component reaction. J Comb Chem 11:341–344. https://doi.org/10.1021/cc800167h
Gozalishvili LL, Beryozkina TV, Omelchenko IV, Zubatyuk RI, Shishkin OV, Kolos NN (2008) A rapid and facile synthesis of new spiropyrimidines from 5-(2-arylethylidene-2-oxo)-1, 3-dimethylpyrimidine-2, 4, 6-triones. Tetrahedron 64:8759–8765. https://doi.org/10.1016/j.tet.2008.06.097
Tinker AC, Beaton HG, Boughton-Smith N, Cook TR, Cooper SL, Fraser-Rae L, Hallam K, Hamley P, McInally T, Nicholls DJ, Pimm AD (2003) 1, 2-dihydro-4-quinazolinamines: potent, highly selective inhibitors of inducible nitric oxide synthase which show antiinflammatory activity in vivo. J Med Chem 46:913–916. https://doi.org/10.1021/jm0255926
Laude EA, Bee D, Crambes O, Howard P (1995) Antitussive and antibronchoconstriction actions of fenspiride in guinea-pigs. Eur Respir J 8:1699–1704. https://doi.org/10.1183/09031936.95.08101699
Chande MS, Verma RS, Barve PA, Khanwelkar RR, Vaidya RB, Ajaikumar KB (2005) Facile synthesis of active antitubercular, cytotoxic and antibacterial agents: a Michael addition approach. Eur J Med Chem 40:1143–1148. https://doi.org/10.1016/j.ejmech.2005.06.004
Behera RK, Behera AK, Pradhan R, Pati A, Patra M (2006) Studies on spiroheterocycles, part II: heterocyclization of the spiro compounds containing cyclohexanone and thiobarbituric acid with different bidentate nucleophilic reagents. Synth Commun 36:3729–3742. https://doi.org/10.1080/00397910600946231
Youssef MM, Amin MA (2010) Microwave assisted synthesis of some new heterocyclic spiro-derivatives with potential antimicrobial and antioxidant activity. Molecules 15:8827–8840. https://doi.org/10.3390/molecules15128827
Oguma T, Katsuki T (2014) Iron-catalyzed asymmetric tandem spiro-cyclization using dioxygen in air as the hydrogen acceptor. Chem Commun 50:5053–5056. https://doi.org/10.1039/c4cc01555j
Huang XF, Zhang YF, Qi ZH, Li NK, Geng ZC, Li K, Wang XW (2014) Organocatalytic enantioselective construction of multi-functionalized spiro oxindole dienes. Org Biomol Chem 12:4372–4385. https://doi.org/10.1039/c4ob00545g
Hu FL, Wei Y, Shi M (2014) Phosphine-catalyzed asymmetric formal [4 + 2] tandem cyclization of activated dienes with isatylidenemalononitriles; enantioselective synthesis of multistereogenic spirocyclic oxindoles. Adv Synth Catal 35(6):736–742. https://doi.org/10.1002/adsc.201301070
Li TZ, **e J, Jiang Y, Sha F, Wu XY (2015) Enantioselective vinylogous Michael/cyclization cascade reaction of acyclic β, γ-unsaturated amides with isatylidene malononitriles: asymmetric construction of spirocyclic oxindoles. Adv Synth Catal 357:3507–3511. https://doi.org/10.1002/adsc.201500469
Li Y, Su X, Zhou W, Li W, Zhang J (2015) Amino acid derived phosphine-catalyzed enantioselective 1, 4-dipolar spiroannulation of cyclobutenones and isatylidenemalononitrile. Chem Eur J 21:4224–4228. https://doi.org/10.1002/chem.201406475
Zhan SC, Sun J, Liu RZ, Yan CG (2020) Diastereoselective construction of carbazole-based spirooxindoles via the Levy three-component reaction. Org Biomol Chem 18:163–168. https://doi.org/10.1039/C9OB02013F
Huang H, Bihani M, Zhao JCG (2016) Stereoselective synthesis of spirooxindole derivatives using an organocatalyzed tandem Michael-Michael reaction. Org Biomol Chem 14:1755–1763. https://doi.org/10.1039/C5OB02348C
Wu H, Wang YM (2014) One-Pot organocatalytic enantioselective Michael/Povarov Domino strategy for the construction of spirooctahydroacridine-3,3′-oxindole scaffolds. Chem Eur J 20:5899–5904. https://doi.org/10.1002/chem.201402002
Chauhan P, Mahajan S, Loh CC, Raabe G, Enders D (2014) Streocontrolled construction of six vicinal stereogenic centers on spiropyrazolones via organocascade Michael/Michael/1, 2-addition reactions. Org Lett 16:2954–2957. https://doi.org/10.1021/ol501093v
Zeng XM, Meng CY, Bao JX, Xu DC, **e JW, Zhu WD (2015) Enantioselective construction of polyfunctionalized spiroannulated dihydrothiophenes via a formal thio [3 + 2] cyclization. J Org Chem 80:11521–11528. https://doi.org/10.1021/acs.joc.5b01357
Saikia UP, Baruah D, Pahari P, Borah MJ, Goswami A, Konwar D (2014) A facile microwave assisted synthesis of spiro-1, 3-oxazines from n-(2-(cyclohex-1-en-1-yl) ethyl) amides. Tetrahedron Lett 55:4328–4330. https://doi.org/10.1016/j.tetlet.2014.06.042
**e YJ, Sun J, Yan CG (2014) Domino reactions of vinyl malononitriles with 3-phenacylideneoxindoles for efficient synthesis of functionalized spirocyclic oxindoles. ACS Comb Sci 16:271–280. https://doi.org/10.1021/co500006c
Chen PQ, **ao YC, Yue CZ, Chen YC (2014) Trienamine catalysis with linear deconjugated 3, 5-dienones. Org Chem Front 1:490–493. https://doi.org/10.1039/C4QO00079J
Tu XC, Yu Y, Tu MS, Jiang B, Li C, Tu SJ (2014) A new iodonium ylide-based three-component reaction leading to 2-spirosubstituted dihydrofurans under microwave irradiation. J Heterocycl Chem 51:436–441. https://doi.org/10.1002/jhet.1732
Batmani H, Noroozi Pesyan N, Havasi F, Aalinejad M (2019) Synthesis of spiro-dihydrofuran in the presence of a novel and reusable nanocatalyst Cu (II)-glycerol/MCM-41. Appl Organomet Chem 33:4997. https://doi.org/10.1002/aoc.4997
Stiller J, Poulsen PH, Cruz DC, Dourado J, Davis RL, Jørgensen KA (2014) Organocatalytic [4 + 2] addition reactions via tetraenamine intermediate. Chem Sci 5:2052–2056. https://doi.org/10.1039/C4SC00081A
Manoni F, Connon SJ (2014) Catalytic asymmetric tamura cycloadditions. Angew Chem Int Ed 53:2628–2632. https://doi.org/10.1002/anie.201309297
Huang LJ, Weng J, Wang S, Lu G (2015) Organocatalytic diels-alder reaction of 2-vinylindoles with methyleneindolinones: an efficient approach to functionalized carbazolespirooxindoles. Adv Synth Catal 357:993–1003. https://doi.org/10.1002/adsc.201400780
**e Y, Que Y, Li T, Zhu L, Yu C, Yao C (2015) N-heterocyclic carbene-catalyzed [4 + 2] cyclization of 2-bromo-2-enal with 3-alkylenyloxindoles: efficient assembly of spirocarbocyclic oxindole. Org Biomol Chem 13:1829–1835. https://doi.org/10.1039/C4OB01706D
Wang B, Leng HJ, Yang XY, Han B, Rao CL, Liu L, Peng C, Huang W (2015) Efficient synthesis of tetrahydronaphthalene or isochroman-fused spirooxindoles using tandem reactions. RSC Adv 5:88272–88276. https://doi.org/10.1039/C5RA15735H
Li ZL, Liu C, Tan R, Tong ZP, Liu YK (2016) Organocatalytic, asymmetric [2 + 2+2] annulation to construct six-membered spirocyclic oxindoles with six continuous stereogenic centers. Catalysts 6:65. https://doi.org/10.3390/catal6050065
Anwar S, Li SM, Chen K (2014) Organocatalytic synthesis of substituted spirocyclohexane carbaldehydes via [4 + 2] annulation strategy between 2-arylideneindane-1, 3-diones and glutaraldehyde. Org Lett 16:2993–2995. https://doi.org/10.1021/ol501160k
Li X, Lin MH, Han Y, Wang F, Cheng JP (2013) Asymmetric diels-alder reaction of 3-olefinic benzofuran-2-ones and polyenals: construction of chiral spirocyclic benzofuran-2-ones. Org Lett 16:114–117. https://doi.org/10.1021/ol403094a
Monleón A, Glaus F, Vergura S, Jørgensen KA (2016) Organocatalytic strategy for the enantioselective cycloaddition to trisubstituted nitroolefins to create spirocyclohexene-oxetane scaffolds. Angew Chem Int Edit 55:2478–2482. https://doi.org/10.1002/anie.201510731
Zhang DY, Xu L, Wu H, Gong LZ (2015) Chiral iodine-catalyzed dearomatizative spirocyclization for the enantioselective construction of an all-carbon stereogenic center. Chem-Eur J 21:10314–10317. https://doi.org/10.1002/chem.201501583
Yang W, Zhang Y, Qiu S, Zhao C, Zhang L, Liu H, Zhou L, **ao Y, Guo H (2015) Phosphine-catalyzed [4 + 2] cycloaddition of unsaturated pyrazolones with allenoates: a concise approach toward spiropyrazolones. RSC Adv 5:62343–62347. https://doi.org/10.1039/C5RA11595G
Meninno S, Mazzanti A, Lattanzi A (2019) Asymmetric synthesis of pyrazolone fused spirocyclohexeneimines via a vinylogous Michael/cyclization cascade reaction. Adv Synth Catal 361:79–84. https://doi.org/10.1002/adsc.201801337
Abbaraju S, Ramireddy N, Rana NK, Arman H, Zhao JCG (2015) Organocatalytic enantioselective synthesis of polysubstituted spirooxindoles using a tandem Michael-Michael reaction. Adv Synth Catal 357:2633–2638. https://doi.org/10.1002/adsc.201500298
Fei J, Qian Q, Sun X, Gu X, Zou C, Ye J (2015) Organocatalytic enantioselective formal [4 + 2] cycloaddition of enones with cyclic N-sulfonylimines and methylene chromene for chiral spirocyclic compounds. Org Lett 17:5296–5299. https://doi.org/10.1021/acs.orglett.5b02667
Gajulapalli VPR, Vinayagam P, Kesavan V (2015) Enantioselective assembly of functionalized carbocyclic spirooxindoles using an l-proline derived thiourea organocatalyst. RSC Adv 5:7370–7379. https://doi.org/10.1039/C4RA13711F
Han B, Huang W, Ren W, He G, Wang JH, Peng C (2015) Asymmetric synthesis of cyclohexane-fused drug-like spirocyclic scaffolds containing six contiguous stereogenic centers via organocatalytic cascade reactions. Adv Synth Catal 357:561–568. https://doi.org/10.1002/adsc.201400764
Yetra SR, Mondal S, Mukherjee S, Gonnade RG, Biju AT (2016) Enantioselective synthesis of spirocyclohexadienones by NHC-catalyzed formal [3 + 3] annulation reaction of enals. Angew Chem Int Ed 55:268–272. https://doi.org/10.1002/anie.201507802
Xu J, Hu L, Hu H, Ge S, Liu X, Feng X (2019) Enantioselective vinylogous Michael-aldol reaction to synthesize spirocyclohexene pyrazolones in aqueous media. Org Lett 21:1632–1636. https://doi.org/10.1021/acs.orglett.9b00168
Amireddy M, Chen K (2016) Organocatalytic one-pot asymmetric synthesis of functionalized spiropyrazolones via a Michael-aldol sequential reaction. RSC Adv 6:77474–77480. https://doi.org/10.1039/C6RA13923J
Yuan Z, Wei W, Lin A, Yao H (2016) Bifunctional organo/metal cooperatively catalyzed [3 + 2] annulation of para-quinone methides with vinylcyclopropanes: approach to spiro [4.5] deca-6, 9-diene-8-ones. Org Lett 18:3370–3373. https://doi.org/10.1021/acs.orglett.6b01512
Rani MA, Kumar SV, Roja SS, Almansour AI, Kumar RS, Athimoolam S, Kumar RR (2016) Synthesis of highly functionalized 2-thiaspiro [4.5] deca-6, 8-dienes via atom efficient tandem Michael addition/Thorpe-Ziegler cyclization. RSC Adv 6:40585–40592. https://doi.org/10.1039/C6RA05572A
Ren W, Wang XY, Li JJ, Tian M, Liu J, Ouyang L, Wang JH (2017) Efficient construction of biologically important functionalized polycyclic spiro-fused carbocyclicoxindoles via an asymmetric organocatalytic quadruple-cascade reaction. RSC Adv 7:1863–1868. https://doi.org/10.1039/C6RA24910H
Beltran F, Andna L, Miesch L (2019) Spirocyclization of keto-ynesulfonamides promoted by quaternary ammonium salts. Org Chem Front 6:373–376. https://doi.org/10.1039/C8QO00937F
Fang W, Wei Y, Shi M (2019) Palladium (II)-catalyzed intermolecular cascade cyclization of methylenecyclopropanes with aromatic alkynes: construction of spirocyclic compounds containing indene and 1, 2-dihydronaphthalene moieties. Adv Synth Catal 361:3446–3450. https://doi.org/10.1002/adsc.201900327
Wang CS, Roisnel T, Dixneuf PH, Soulé JF (2019) Access to 3-(2-oxoalkyl)-azaspiro [4.5] trienones via acid-triggered oxidative cascade reaction through alkenyl peroxide radical intermediate. Adv Synth Catal 361:445–450. https://doi.org/10.1002/adsc.201801203
Guo S, Liu Y, Zhao L, Zhang X, Fan X (2019) Rhodium-catalyzed selective oxidative (spiro) annulation of 2-arylindoles by using benzoquinone as a C2 or C1 synthon. Org Lett 21:6437–6441. https://doi.org/10.1021/acs.orglett.9b02336
Han Y, ** Y, Jiang M, Yang H, Fu H (2019) Photocatalyst-free visible-light photoredox dearomatization of phenol derivatives containing ketoximes: an easy access to spiropyrrolines. Org Lett 21:1799–1803. https://doi.org/10.1021/acs.orglett.9b00372
Gao Y, Liu D, Fu Z, Huang W (2019) Facile synthesis of 2, 2-diacyl spirocyclohexanones via an N-heterocyclic carbene-catalyzed formal [3C + 3C] annulation. Org Lett 21:926–930. https://doi.org/10.1021/acs.orglett.8b03892
Ni J, Jiang Y, Qi Z, Yan R (2019) TFAA-catalyzed annulation synthesis of spiro pyrrolo [1, 2-a] quinoxaline derivatives from 1-(2-aminophenyl) pyrroles and benzoquinones/ketones. Chem Asian J 14:2898–2902. https://doi.org/10.1002/asia.201900567
Liu XL, Zuo X, Wang JX, Chang SQ, Wei QD, Zhou Y (2019) A bifunctional pyrazolone-chromone synthon directed organocatalytic double Michael cascade reaction: forging five stereocenters in structurally diverse hexahydroxanthones. Org Chem Front 6:1485–1490. https://doi.org/10.1039/C9QO00265K
Zhang M, Wang JX, Chang SQ, Liu XL, Zuo X, Zhou Y (2019) Highly efficient enantioselective synthesis of bispiro [benzofuran-oxindole/benzofuran-chromanone]s through organocatalytic inter-/intramolecular Michael cycloaddition. Chin Chem Lett. https://doi.org/10.1016/j.cclet.2019.06.015
Sarkar D, Rout N (2019) Ruthenium (VIII)-catalyzed ipso-dearomative spiro-etherification and spiro-amidation of phenols. Org Lett 21:4132–4136. https://doi.org/10.1021/acs.orglett.9b01322
Hsu DS, Cheng CY (2019) Construction of spirofused tricyclic frameworks by NHC-catalyzed intramolecular Stetter reaction of a benzaldehyde tether with a cyclic enone. J Org Chem 84:10832–10842. https://doi.org/10.1021/acs.joc.9b01403
Elagamy A, Shaw R, Panwar R, Shally Ram VJ, Pratap R (2019) Synthesis of highly functionalized spirobutenolides via a nitroalkane-mediated ring contraction of 2-oxobenzo [h] chromenes through denitration. J Org Chem 84:154–1161. https://doi.org/10.1021/acs.joc.8b02257
Zhou L, **a Y, Wang YZ, Fang JD, Liu XY (2019) Mn (III)-Promoted synthesis of spiroannular tricyclic scaffolds via sulfonylation/dearomatization of biaryl ynones. Tetrahedron 75:1267–1274. https://doi.org/10.1016/j.tet.2019.01.041
Karadeniz E, Zora M (2019) Synthesis of 1-azaspiro [4.5] deca-1, 3-dienes from N-propargylic β-enaminones in basic medium. Synthesis 51:2157–2170. https://doi.org/10.1055/s-0037-1611723
Almansour A, Kumar R, Beevi F, Shirazi A, Osman H, Ismail R, Choon T, Sullivan B, McCaffrey K, Nahhas A, Parang K (2014) Facile, regio-and diastereoselective synthesis of spiro-pyrrolidine and pyrrolizine derivatives and evaluation of their antiproliferative activities. Molecules 19:10033–10055. https://doi.org/10.3390/molecules190710033
Rambla M, Duroure L, Chabaud L, Guillou C (2014) Enantioselective synthesis of spiroimines by asymmetric decarboxylative alkylation/isomerization/[3 + 2]-cycloaddition reaction of azidoalkenes. Eur J Org Chem 2014:7716–7720. https://doi.org/10.1002/ejoc.201403161
Goudedranche S, Bugaut X, Constantieux T, Bonne D, Rodriguez J (2014) α, β-Unsaturated acyl cyanides as new bis-electrophiles for enantioselective organocatalyzed formal [3 + 3] spiroannulation. Chem-Eur J 20:410–415. https://doi.org/10.1002/chem.201303613
Dai W, Lu H, Li X, Shi F, Tu SJ (2014) Diastereo-and enantioselective construction of a bispirooxindole scaffold containing a tetrahydro-β-carboline moiety through an organocatalytic asymmetric cascade reaction. Chem Eur J 20:11382–11389. https://doi.org/10.1002/chem.201402485
Bhuyan D, Sarmah MM, Dommaraju Y, Prajapati D (2014) Microwave-promoted efficient synthesis of spiroindenotetrahydropyridine derivatives via a catalyst-and solvent-free pseudo one-pot five-component tandem Knoevenagel/aza-diels-alder reaction. Tetrahedron Lett 55:5133–5136. https://doi.org/10.1016/j.tetlet.2014.07.086
Tejedor D, Cotos L, Méndez-Abt G, García-Tellado F (2014) General synthesis of substituted 1, 2-dihydropyridines. J Org Chem 79:10655–10661. https://doi.org/10.1021/jo501991s
Faty R, Rashed M, Youssef M (2015) Microwave-assisted synthesis and antimicrobial evaluation of novel spiroisoquinoline and spiropyrido [4, 3-d] pyrimidine derivatives. Molecules 20:1842–1859. https://doi.org/10.3390/molecules20021842
Zheng H, Liu X, Xu C, **a Y, Lin L, Feng X (2015) Regio-and enantioselective aza-Diels–Alder reactions of 3-vinylindoles: a concise synthesis of the antimalarial spiroindolone NITD609. Angew Chem Int Edit 54:10958–10962. https://doi.org/10.1002/anie.201505717
Alizadeh A, Moafi L (2015) An efficient synthesis of spiro-oxindole derivatives by three-component reactions in water. Helv Chim Acta 98:546–551. https://doi.org/10.1002/hlca.201400263
Poomathi N, Mayakrishnan S, Muralidharan D, Srinivasan R, Perumal PT (2015) Reaction of isatins with 6-amino uracils and isoxazoles: isatin ring-opening vs. annulations and regioselective synthesis of isoxazole fused quinoline scaffolds in water. Green Chem 17:3362–3372. https://doi.org/10.1039/C5GC00006H
Rahimi F, Bayat M, Hosseini H (2019) Synthesis of spiroimidazopyridineoxindole, spiropyridopyrimidineoxindole and spiropyridodiazepineoxindole derivatives based on heterocyclic ketene aminals via a four-component reaction. RSC Adv 9:16384–16389. https://doi.org/10.1039/C8RA10379H
**e D, Yang L, Lin Y, Zhang Z, Chen D, Zeng X, Zhong G (2015) Rapid access to spirocylic oxindoles: application of asymmetric n-heterocyclic carbene-catalyzed [3 + 3] cycloaddition of imines to oxindole-derived enals. Org Lett 17:2318–2321. https://doi.org/10.1021/acs.orglett.5b00726
You Y, Cui BD, Zhou MQ, Zuo J, Zhao JQ, Xu XY, Zhang XM, Yuan WC (2015) Organocatalytic asymmetric Michael/Friedel-Crafts cascade reaction of 3-pyrrolyl-oxindoles and α, β-unsaturated aldehydes for the construction of chiral spiro [5, 6-dihydropyrido [1, 2-a] pyrrole-3, 3′-oxindoles]. J Org Chem 80:5951–5957. https://doi.org/10.1021/acs.joc.5b00597
Chidurala P, Jetti V, Meshram JS (2016) Facile synthesis of new 3, 5-dispiro substituted piperidine analogs via microwave-assisted one pot multicomponent reaction. J Heterocycl Chem 53:389–392. https://doi.org/10.1002/jhet.2424
Patil A, Salunkhe R (2018) Metal free green protocol for the synthesis of bis-spiro piperidine and pyrimidine derivatives. Res Chem Intermediat 44:3337–3348. https://doi.org/10.1007/s11164-018-3310-7
Safari E, Maryamabadi A, Hasaninejad B (2017) A highly efficient, one-pot synthesis of novel bis-spirooxindoles with skeletal diversity via sequential multi-component reaction in PEG-400 as a biodegradable solvent. RSC Adv 7:39502–39511. https://doi.org/10.1039/C7RA06017C
Ghandi M, Ahangaran MM, Abbasi A (2017) Sequential one-pot five-component synthesis of tetrazole-based spirotetrahydro-β-carbolines. J Iran Chem Soc 14:1131–1137. https://doi.org/10.1007/s13738-017-1063-7
Peneau A, Retailleau P, Guillou C, Chabaud L (2018) Rhodium (III)-catalyzed synthesis of spiropiperidine derivatives via C-H activation. J Org Chem 83:2324–2340. https://doi.org/10.1021/acs.joc.7b03252
He C, Li Z, Xu J, Ren H (2019) Asymmetric synthesis of spirocyclic oxindole δ-lactams via NHC-catalyzed formal [2 + 4] annulation of aliphatic aldehydes with oxindole-derived α, β-unsaturated ketimines. J Org Chem 84:12177–12186. https://doi.org/10.1021/acs.joc.9b01760
Martyloga OV, Myronenko A, Tkachenko AM, Matvienko VO, Kuchkovska YO, Grygorenko OO (2019) Multigram synthesis of functionalized spirocyclic diazirines. Eur J Org Chem 2019:3744–3750. https://doi.org/10.1002/ejoc.201900485
Mirhosseini-Eshkevari B, Ghasemzadeh MA, Esnaashari M (2019) Highly efficient and green approach for the synthesis of spirooxindole derivatives in the presence of novel Brønsted acidic ionic liquids incorporated in UiO-66 nanocages. Appl Organomet Chem 33:5027. https://doi.org/10.1002/aoc.5027
Wang Z, Niu J, Zeng H, Li CJ (2019) Construction of spirocyclic tetrahydro-β-carbolines via cross-annulation of phenols with tryptamines in water. Org Lett 21:7033–7037. https://doi.org/10.1021/acs.orglett.9b02613
Yuan H, Tang C, Su S, Cui L, Jia X, Li C, Li J (2019) A bicyclization reaction with two molecular allenyl ketones and isocyanides: synthesis of a lactone-containing azaspirocycle derivative. Chem Comm 55:7231–7234. https://doi.org/10.1039/C9CC02785H
Kong L, Huang R, He H, Fan Y, Lin J, Yan S (2020) Multi-component solvent-free cascade reaction of 2-cyanoacetamides: regioselective synthesis of pyridin-2-ones bearing quaternary centers. Green Chem. https://doi.org/10.1039/c9gc03692j
**e X, Peng C, Leng HJ, Wang B, Tang ZW, Huang W (2014) Asymmetric synthesis of oxa-spirocyclic indanones with structural complexity via an organocatalytic Michael-Henry-acetalization cascade. Synlett 25:143–147. https://doi.org/10.1055/s-0033-1340077
Gao TP, Lin JB, Hu XQ, Xu PF (2014) A catalytic asymmetric hetero-diels-alder reaction of olefinic azlactones and isatins: facile access to chiral spirooxindole dihydropyranones. Chem Commun 50:8934–8936. https://doi.org/10.1039/C4CC03896G
**ao Z, Yu C, Li T, Wang XS, Yao C (2014) N-heterocyclic carbene/Lewis acid strategy for the stereoselective synthesis of spirocyclic oxindole-dihydropyranones. Org Lett 16:3632–3635. https://doi.org/10.1021/ol501224p
Cui HL, Chouthaiwale PV, Yin F, Tanaka F (2016) Catalytic asymmetric hetero-diels-alder reactions of enones with isatins to access functionalized spirooxindole tetrahydropyrans: scope, derivatization, and discovery of bioactives. Org Biomol Chem 14:1777–1783. https://doi.org/10.1039/C5OB02393A
Lin Y, Yang L, Deng Y, Zhong G (2015) Cooperative catalysis of N-heterocyclic carbene and Brønsted acid for a highly enantioselective route to unprotected spiro-indoline-pyrans. Chem Commun 51:8330–8333. https://doi.org/10.1039/C5CC02096D
Parthasarathy K, Praveen C, Jeyaveeran JC, Prince AAM (2016) Gold catalyzed double condensation reaction: synthesis, antimicrobial and cytotoxicity of spirooxindole derivatives. Bioorg Med Chem Lett 26:4310–4317. https://doi.org/10.1016/j.bmcl.2016.07.036
Ghadiri S, Bayat M, Hosseini FS (2019) A simple and environmentally benign synthesis of novel spiro [indoline-3, 5′-pyrano [2, 3-d] pyrimidine] derivatives in water. Monatsh Chem 150:1079–1084. https://doi.org/10.1007/s00706-019-2356-6
Zhao HW, Li B, Tian T, Meng W, Yang Z, Song XQ, Chen XQ, Pang HL (2015) Highly enantioselective synthesis of chiral pyranonaphthoquinone-fused spirooxindoles through organocatalytic three-component cascade reactions. Eur J Org Chem 2015:3320–3326. https://doi.org/10.1002/ejoc.201500152
Mohamadpour F, Maghsoodlou MT, Heydari R, Lashkari M (2016) Copper (II) acetate monohydrate: an efficient and eco-friendly catalyst for the one-pot multi-component synthesis of biologically active spiropyrans and 1h-pyrazolo [1, 2-b] phthalazine-5, 10-dione derivatives under solvent-free conditions. Res Chem Intermediat 42:7841–7853. https://doi.org/10.1007/s11164-016-2565-0
Rezvanian A, Zadsirjan V, Saedi P, Heravi MM (2018) Iodine-catalyzed one-pot four-component synthesis of spiro [indoline-3, 4′-pyrano-pyrazole] derivatives. J Heterocycl Chem 55:2772–2780. https://doi.org/10.1002/jhet.3342
Meshram G, Wagh P, Amratlal V, Deshpande S (2015) Efficient microwave-assisted one-pot synthesis of novel spironaphthopyrano-one derivatives. J Heterocycl Chem 52:1639–1645. https://doi.org/10.1002/jhet.2274
Nasab NH, Safari J (2019) Synthesis of a wide range of biologically important spiropyrans and spiroacenaphthylenes, using NiFe2O4@SiO2@ melamine magnetic nanoparticles as an efficient, green and reusable nanocatalyst. J Mol Struct 1193:118–124. https://doi.org/10.1016/j.molstruc.2019.05.023
Zhu L, Chen Q, Shen D, Zhang W, Shen C, Zeng X, Zhong G (2016) Enantioselective construction of spirocyclic oxindole derivatives with multiple stereocenters via an organocatalytic Michael/aldol/hemiacetalization cascade reaction. Org Lett 18:2387–2390. https://doi.org/10.1021/acs.orglett.6b00873
Heravi MM, Hashemi E, Azimian F (2015) N-sulfonic acid modified poly (styrene-co-maleic anhydride): an efficient and recyclable solid acid catalyst for the synthesis of a wide range of spiropyrans. J Iran Chem Soc 12:647–653. https://doi.org/10.1007/s13738-014-0523-6
Ashok D, Gandhi DM, Kumar AV, Srinivas G, Reddy MS, Kanth SS, Vijjulatha M (2016) Microwave assisted synthesis, biological evaluation, and molecular docking of novel chroman scaffolds incorporating spirochromanone framework. Med Chem Res 25:2882–2899. https://doi.org/10.1007/s00044-016-1699-3
Yazdani-Elah-Abadi A, Maghsoodlou MT, Mohebat R, Heydari R (2017) Theophylline as a new and green catalyst for the one-pot synthesis of spiro [benzo [a] pyrano [2, 3-c] phenazine] and benzo [α] pyrano [2, 3-c] phenazine derivatives under solvent-free conditions. Chin Chem Lett 28:446–452. https://doi.org/10.1016/j.cclet.2016.09.016
Mohebat R, Simin N, Yazdani-Elah-Abadi AA (2019) Rapid and highly efficient microwave-promoted four-component domino reaction for the synthesis of novel spiro [benzo [a] chromeno [2, 3-c] phenazine] derivatives under solvent-free conditions. Polycycl Aromat Compd 39:148–158. https://doi.org/10.1080/10406638.2017.1293698
Muthusamy S, Prakash M, Ramakrishnan C, Gromiha MM, Kesavan V (2016) Organocatalytic enantioselective assembly of spirooxindole-naphthopyrans through tandem Friedel-Crafts type/hemiketalization. ChemCatChem 8:1708–1712. https://doi.org/10.1002/cctc.201600087
Yin SJ, Zhang SY, Zhang JQ, Sun BB, Fan WT, Wu B, Wang XW (2016) Organocatalytic tandem enantioselective Michael-cyclization of isatin-derived β, γ-unsaturated α-ketoesters with 3-hydroxy-4 h-chromen-4-one or 2-hydroxy-1, 4-naphthoquinone derivatives. RSC Adv 6:84248–84254. https://doi.org/10.1039/C6RA17400K
Guranova NI, Darin D, Kantin G, Novikov AS, Bakulina O, Krasavin M (2019) Rh (II)-catalyzed spirocyclization of α-diazo homophthalimides with cyclic ethers. J Org Chem 84:4534–4542. https://doi.org/10.1021/acs.joc.9b00245
Afeke C, **e Y, Floreancig PE (2019) Re2O7-catalyzed approach to spirocyclic ether formation from acyclic precursors: observation of remote stereoinduction. Org Lett 21:5064–5067. https://doi.org/10.1021/acs.orglett.9b01660
Shen YB, Li SS, Liu X, Yu L, Sun YM, Liu Q, **ao J (2019) Formal [4 + 2] annulation of oxindole-embedded ortho-quinone methides with 1, 3-dicarbonyls: synthesis of spiro [chromen-4, 3′-oxindole] scaffolds. J Org Chem 84:3990–3999. https://doi.org/10.1021/acs.joc.8b03260
Tamura R, Kitamura E, Tsutsumi R, Yamanaka M, Akiyama T, Mori K (2019) Diastereoselective synthesis of CF3-substituted spiroisochromans by [1, 5]-hydride shift/cyclization/intramolecular Friedel-Crafts reaction sequence. Org Lett 21:2383–2387. https://doi.org/10.1021/acs.orglett.9b00668
Yang W, Wang X, ** X, Sun H, Guo R, Xu W, Cai Q (2019) Copper-catalysed double O-arylation for enantioselective synthesis of oxa-spirocycles. Adv Synth Catal 361:562–568. https://doi.org/10.1002/adsc.201801395
Liu J, Tian Y, Shi J, Zhang S, Cai Q (2015) An enantioselective synthesis of spirobilactams through copper-catalyzed intramolecular double N-arylation and phase separation. Angew Chem 127:11067–11070. https://doi.org/10.1002/anie.201504589
Cao W, Liu X, Guo J, Lin L, Feng X (2015) Asymmetric tandem 1, 5-hydride shift/ring closure for the synthesis of chiral spirooxindole tetrahydroquinolines. Chem Eur J 21:1632–1636. https://doi.org/10.1002/chem.201404327
Zhu QN, Zhang YC, Xu MM, Sun XX, Yang X, Shi F (2016) Enantioselective construction of tetrahydroquinolin-5-one-based spirooxindole scaffold via an organocatalytic asymmetric multicomponent [3 + 3] cyclization. J Org Chem 8:7898–7907. https://doi.org/10.1021/acs.joc.6b01598
Kong DL, Lu GP, Wu MS, Shi ZF, Lin Q (2017) One-pot, catalyst-free synthesis of spiro [dihydroquinoline-naphthofuranone] compounds from isatins in water triggered by hydrogen bonding effects. ACS Sustain Chem Eng 5:3465–3470. https://doi.org/10.1021/acssuschemeng.7b00145
Gill CH, Chate AV, Shinde GY, Sarkate AP, Tiwari SV (2018) One-pot, four-component synthesis and SAR STUDIES of spiro [pyrimido [5, 4-b] quinoline-10, 5′-pyrrolo [2, 3-d] pyrimidine] derivatives catalyzed by β-cyclodextrin in water as potential anticancer agents. Res Chem Intermediat 44:4029–4043. https://doi.org/10.1007/s11164-018-3353-9
Li SS, Zhu S, Chen C, Duan K, Liu Q, **ao J (2019) Hydride transfer involved redox-neutral cascade cyclizations for construction of spirocyclic bisoxindoles featuring a [3, 4]-fused oxindole moiety. Org Lett 21:1058–1062. https://doi.org/10.1021/acs.orglett.8b04100
Josa-Culleré L, Hirst MG, Lockett JP, Thompson AL, Moloney MG (2019) Spirocyclic tetramates by sequential knoevenagel and [1, 5]-prototropic shift. J Org Chem 84:9671–9683. https://doi.org/10.1021/acs.joc.9b01345
Lv X, Hu F, Duan K, Li SS, Liu Q, **ao J (2019) Aromatization-driven cascade [1, 5]-hydride transfer/spirocyclization promoted by fluorinated alcohols. J Org Chem 84:1833–1844. https://doi.org/10.1021/acs.joc.8b02754
Zhao HW, Tian T, Li B, Yang Z, Pang HL, Meng W, Song XQ, Chen XQ (2015) Diastereoselective synthesis of dispirobarbiturates through et3n-catalyzed [3 + 2] cycloaddition of barbiturate-based olefins with 3-isothiocyanato oxindoles. J Org Chem 80:10380–10385. https://doi.org/10.1021/acs.joc.5b01810
Liu H, Liu Y, Yuan C, Wang GP, Zhu SF, Wu Y, Wang B, Sun Z, **ao Y, Zhou QL, Guo H (2016) Enantioselective synthesis of spirobarbiturate-cyclohexenes through phosphine-catalyzed asymmetric [4 + 2] annulation of barbiturate-derived alkenes with allenoates. Org Lett 18:1302–1305. https://doi.org/10.1021/acs.orglett.6b00239
Krasnov KA, Dorovatovskii PV, Zubavichus YV, Timofeeva TV, Khrustalev VN (2017) Hydride transfer reactions of 5-(2-alkohybenzylidene) barbituric acids: synthesis of 2, 4, 6-trioxoperhydropyrimidine-5-spiro-3′-chromanes. Tetrahedron 73:542–549. https://doi.org/10.1016/j.tet.2016.12.045
Platonova AY, Glukhareva TV, Zimovets OA, Morzherin YY (2013) Tert-amino effect: the Meth-Cohn and Reinhoudt reactions. Chem Heterocycl Compd 49:357–385. https://doi.org/10.1007/s10593-013-1257-6
Nagaraju S, Sathish K, Paplal B, Kashinath D (2017) “On-water” catalyst-free, one-pot synthesis of quaternary centered and spiro-tetrahydrothiophene-barbiturate hybrids. Tetrahedron Lett 58:2865–2871. https://doi.org/10.1016/j.tetlet.2017.06.029
Rajeswari M, Saluja P, Khurana JM (2016) A facile and green approach for the synthesis of spiro [naphthalene-2, 5′-pyrimidine]-4-carbonitrile via a one-pot three-component condensation reaction using DBU as a catalyst. RSC Adv 6:1307–1312. https://doi.org/10.1039/C5RA22817D
Lohar T, Kumbhar A, Patil A, Kamat S, Salunkhe R (2019) Synthesis and characterization of new quaternary ammonium surfactant [C 18-dabco][Br] and its catalytic application in the synthesis of spirocarbocycles under ultrasonic condition. Res Chem Intermediat 45:1639–1651. https://doi.org/10.1007/s11164-018-3690-8
Pakravan N, Shayani-Jam H, Beiginejad H, Tavafi H, Paziresh S (2019) A green method for the synthesis of novel spiro compounds: enhancement of antibacterial properties of caffeic acid through electrooxidation in the presence of barbituric acid derivatives. J Electroanal Chem 848:113286. https://doi.org/10.1016/j.jelechem.2019.113286
Miklós F, Petrisor A, Fülöp F (2015) Alternative conditions for the synthesis of novel spiro [1, 3-N, N-heterocyclic-adamantanes]. Arkivoc 2015:158–171. https://doi.org/10.3998/ark.5550190.p009.268
Stucchi M, Lesma G, Meneghetti F, Rainoldi G, Sacchetti A, Silvani A (2016) organocatalytic asymmetric Biginelli-like reaction involving isatin. J Org Chem 81:1877–1884. https://doi.org/10.1021/acs.joc.5b02680
Kalogirou AS, Kourtellaris A, Koutentis PA (2019) Synthesis and reactivity of 3′, 5′-dichloro-1H-spiro (quinazoline-2, 4′-[1, 2, 6] thiadiazin)-4 (3H)-ones. Eur J Org Chem 2019:5462–5474. https://doi.org/10.1002/ejoc.201900576
Zali-Boeini H, Karimi F, Khayat Z, Rudbari HA, Abbasi A (2019) A novel one-pot three-component approach to 4-amino-functionalized spiropyrimidine-2-thiones. J Iran Chem Soc 16:1139–1146. https://doi.org/10.1007/s13738-018-01589-9
Engen K, Savmarker J, Rosenstrom U, Wannberg J, Lundback T, Jenmalm-Jensen A, Larhed M (2014) Microwave heated flow synthesis of spiro-oxindole dihydroquinazolinone based IRAP inhibitors. Org Process Res Dev 18:1582–1588. https://doi.org/10.1021/op500237k
Chen X, Zhang JQ, Yin SJ, Li HY, Zhou WQ, Wang XW (2015) Asymmetric construction of spiro [thiopyranoindole-benzoisothiazole] scaffold via a formal [3 + 3] spiroannulation. Org Lett 17:4188–4191. https://doi.org/10.1021/acs.orglett.5b01951
Wang S, Jiang Y, Wu S, Dong G, Miao Z, Zhang W, Sheng C (2016) Meeting organocatalysis with drug discovery: asymmetric synthesis of 3, 3′-spirooxindoles fused with tetrahydrothiopyrans as novel p53-MDM2 inhibitors. Org Lett 18:1028–1031. https://doi.org/10.1021/acs.orglett.6b00155
Maloo P, Roy TK, Sawant DM, Pardasani RT, Salunkhe MM (2016) A catalyst-free, one-pot multicomponent synthesis of spiro-benzimidazoquinazolinones via a knoevenagel-Michael-imine pathway: a microwave assisted approach. RSC Adv 6:41897–41906. https://doi.org/10.1039/C6RA05322J
Faltracco M, Cotogno S, Vande Velde CM, Ruijter E (2019) Catalytic asymmetric synthesis of diketopiperazines by intramolecular Tsuji-Trost allylation. J Org Chem 84:12058–12070. https://doi.org/10.1021/acs.joc.9b01994
Santra S, Andreana PR (2011) A rapid, one-pot, microwave-influenced synthesis of spiro-2,5-diketopiperazines via a cascade Ugi/6-exo-trig aza-Michael reaction. J Org Chem 76:2261–2264. https://doi.org/10.1021/jo102305q
Acknowledgements
The authors are thankful to GC University, Faisalabad, for providing the facilities to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Babar, K., Zahoor, A.F., Ahmad, S. et al. Recent synthetic strategies toward the synthesis of spirocyclic compounds comprising six-membered carbocyclic/heterocyclic ring systems. Mol Divers 25, 2487–2532 (2021). https://doi.org/10.1007/s11030-020-10126-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-020-10126-x