Abstract
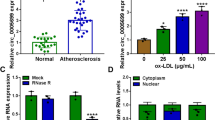
Cerebrovascular diseases have extreme high mortality and disability rate worldwide, and endothelial cells injury-induced atherosclerosis acts as the main cause of cerebrovascular disease. Ferroptosis is a novel type of programmed cell death depending on iron-lipid peroxidation. Recent studies have revealed that ferroptosis might promote the progression of atherosclerosis (AS). Here, this research aimed to investigate the function and its profound mechanism on vascular endothelial cells in atherosclerosis. Research results revealed that YTHDF2 expression up-regulated in ox-LDL treated human umbilical vein endothelial cells (HUVECs). Gain/loss functional assays indicated that YTHDF2 overexpression inhibited HUVECs’ proliferation and accelerated the ferroptosis in ox-LDL-administered HUVECs. Meanwhile, YTHDF2 silencing promoted cell proliferation and reduced the ferroptosis in ox-LDL-administered HUVECs. Mechanistically, in silico analysis suggested that there were potential m6A-modified sites on SLC7A11 mRNA, and YTHDF2 could bind with SLC7A11 mRNA via m6A-dependent manner. YTHDF2 promoted the degradation of SLC7A11 mRNA, thereby reducing its mRNA stability. Taken together, these findings suggest that YTHDF2 accelerates endothelial cells ferroptosis in cerebrovascular atherosclerosis, hel** us enhance our comprehension on cerebrovascular disease pathological physiology.





Similar content being viewed by others
Data availability
No research data shared.
References
Caplan LR, Searls DE, Hon FK (2009) Cerebrovascular disease. Med Clin N Am 93:353–369, viii. https://doi.org/10.1016/j.mcna.2008.09.004
Johansen MC, Gottesman RF (2021) Cerebrovascular disease and cognitive outcome in patients with cardiac disease. Semin Neurol 41:463–472. https://doi.org/10.1055/s-0041-1726330
Miller EC (2019) Preeclampsia and cerebrovascular disease. Hypertension 74:5–13. https://doi.org/10.1161/hypertensionaha.118.11513
Narasimhan M, Schwartz R, Halliday G (2022) Parkinsonism and cerebrovascular disease. J Neurol Sci 433:120011. https://doi.org/10.1016/j.jns.2021.120011
Osorio RC, Oh JY, Choudhary N, Lad M, Savastano L, Aghi MK (2022) Pituitary adenomas and cerebrovascular disease: a review on pathophysiology, prevalence, and treatment. Front Endocrinol (Lausanne) 13:1064216. https://doi.org/10.3389/fendo.2022.1064216
Santos M, de Sousa DA (2022) Cerebrovascular disease in pregnancy and postpartum. Curr Opin Neurol 35:31–38. https://doi.org/10.1097/wco.0000000000001005
Chen X, Li J, Kang R, Klionsky DJ, Tang D (2021) Ferroptosis: machinery and regulation. Autophagy 17:2054–2081. https://doi.org/10.1080/15548627.2020.1810918
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, Sun B, Wang G (2020) Ferroptosis: past, present and future. Cell Death Dis 11:88. https://doi.org/10.1038/s41419-020-2298-2
Bano I, Horky P, Abbas SQ, Majid M, Bilal AHM, Ali F, Behl T, Hassan SSU, Bungau S (2022) Ferroptosis: a new road towards cancer management. Molecules. https://doi.org/10.3390/molecules27072129
Mou Y, Wang J, Wu J, He D, Zhang C, Duan C, Li B (2019) Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol 12:34. https://doi.org/10.1186/s13045-019-0720-y
Tang D, Chen X, Kang R, Kroemer G (2021) Ferroptosis: molecular mechanisms and health implications. Cell Res 31:107–125. https://doi.org/10.1038/s41422-020-00441-1
Yan HF, Zou T, Tuo QZ, Xu S, Li H, Belaidi AA, Lei P (2021) Ferroptosis: mechanisms and links with diseases. Signal Transduct Target Ther 6:49. https://doi.org/10.1038/s41392-020-00428-9
Hou Y, Zhang Q, Pang W, Hou L, Liang Y, Han X, Luo X, Wang P, Zhang X, Li L, Meng X (2021) YTHDC1-mediated augmentation of miR-30d in repressing pancreatic tumorigenesis via attenuation of RUNX1-induced transcriptional activation of Warburg effect. Cell Death Differ 28:3105–3124. https://doi.org/10.1038/s41418-021-00804-0
Hu Y, Ouyang Z, Sui X, Qi M, Li M, He Y, Cao Y, Cao Q, Lu Q, Zhou S, Liu L, Liu L, Shen B, Shu W, Huo R (2020) Oocyte competence is maintained by m(6)A methyltransferase KIAA1429-mediated RNA metabolism during mouse follicular development. Cell Death Differ 27:2468–2483. https://doi.org/10.1038/s41418-020-0516-1
Li ZX, Zheng ZQ, Yang PY, Lin L, Zhou GQ, Lv JW, Zhang LL, Chen F, Li YQ, Wu CF, Li F, Ma J, Liu N, Sun Y (2022) WTAP-mediated m(6)A modification of lncRNA DIAPH1-AS1 enhances its stability to facilitate nasopharyngeal carcinoma growth and metastasis. Cell Death Differ 29:1137–1151. https://doi.org/10.1038/s41418-021-00905-w
Dong G, Yu J, Shan G, Su L, Yu N, Yang S (2021) N6-methyladenosine methyltransferase METTL3 promotes angiogenesis and atherosclerosis by upregulating the JAK2/STAT3 pathway via m6A reader IGF2BP1. Front Cell Dev Biol 9:731810. https://doi.org/10.3389/fcell.2021.731810
Shen H, **e K, Tian Y, Wang X (2023) N6-methyladenosine writer METTL3 accelerates the sepsis-induced myocardial injury by regulating m6A-dependent ferroptosis. Apoptosis 28:514–524. https://doi.org/10.1007/s10495-022-01808-y
Xu Y, Lv D, Yan C, Su H, Zhang X, Shi Y, Ying K (2022) METTL3 promotes lung adenocarcinoma tumor growth and inhibits ferroptosis by stabilizing SLC7A11 m(6)A modification. Cancer Cell Int 22:11. https://doi.org/10.1186/s12935-021-02433-6
Gao Z, Li C, Sun H, Bian Y, Cui Z, Wang N, Wang Z, Yang Y, Liu Z, He Z, Li B, Li F, Li Z, Wang L, Zhang D, Yang L, Xu Z, Li X, Xu H (2023) N(6)-methyladenosine-modified USP13 induces pro-survival autophagy and imatinib resistance via regulating the stabilization of autophagy-related protein 5 in gastrointestinal stromal tumors. Cell Death Differ 30:544–559. https://doi.org/10.1038/s41418-022-01107-8
Ji X, Liu Z, Gao J, Bing X, He D, Liu W, Wang Y, Wei Y, Yin X, Zhang F, Han M, Lu X, Wang Z, Liu Q, **n T (2023) N(6)-methyladenosine-modified lncRNA LINREP promotes glioblastoma progression by recruiting the PTBP1/HuR complex. Cell Death Differ 30:54–68. https://doi.org/10.1038/s41418-022-01045-5
Liu HT, Zou YX, Zhu WJ, Sen L, Zhang GH, Ma RR, Guo XY, Gao P (2022) lncRNA THAP7-AS1, transcriptionally activated by SP1 and post-transcriptionally stabilized by METTL3-mediated m6A modification, exerts oncogenic properties by improving CUL4B entry into the nucleus. Cell Death Differ 29:627–641. https://doi.org/10.1038/s41418-021-00879-9
Chen J, Li X, Ge C, Min J, Wang F (2022) The multifaceted role of ferroptosis in liver disease. Cell Death Differ 29:467–480. https://doi.org/10.1038/s41418-022-00941-0
Rong Y, Fan J, Ji C, Wang Z, Ge X, Wang J, Ye W, Yin G, Cai W, Liu W (2022) USP11 regulates autophagy-dependent ferroptosis after spinal cord ischemia–reperfusion injury by deubiquitinating Beclin 1. Cell Death Differ 29:1164–1175. https://doi.org/10.1038/s41418-021-00907-8
Wang Y, Yan S, Liu X, Deng F, Wang P, Yang L, Hu L, Huang K, He J (2022) PRMT4 promotes ferroptosis to aggravate doxorubicin-induced cardiomyopathy via inhibition of the Nrf2/GPX4 pathway. Cell Death Differ 29:1982–1995. https://doi.org/10.1038/s41418-022-00990-5
Yu W, Liu W, **e D, Wang Q, Xu C, Zhao H, Lv J, He F, Chen B, Yamamoto T, Koyama H, Cheng J (2022) High level of uric acid promotes atherosclerosis by targeting NRF2-mediated autophagy dysfunction and ferroptosis. Oxid Med Cell Longev 2022:9304383. https://doi.org/10.1155/2022/9304383
Bao X, Luo X, Bai X, Lv Y, Weng X, Zhang S, Leng Y, Huang J, Dai X, Wang Y, Li J, Jia H (2023) Cigarette tar mediates macrophage ferroptosis in atherosclerosis through the hepcidin/FPN/SLC7A11 signaling pathway. Free Radic Biol Med 201:76–88. https://doi.org/10.1016/j.freeradbiomed.2023.03.006
Zhang X, Li X, Jia H, An G, Ni J (2021) The m(6)A methyltransferase METTL3 modifies PGC-1α mRNA promoting mitochondrial dysfunction and oxLDL-induced inflammation in monocytes. J Biol Chem 297:101058. https://doi.org/10.1016/j.jbc.2021.101058
Funding
No.
Author information
Authors and Affiliations
Contributions
Jia Li, Changlin Zou, Zhiming Zhang performed the assays and wrote the main manuscript and prepared figures 1-5. Feng Xue was responsible for the funding. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Zou, C., Zhang, Z. et al. N6-methyladenosine (m6A) reader YTHDF2 accelerates endothelial cells ferroptosis in cerebrovascular atherosclerosis. Mol Cell Biochem (2023). https://doi.org/10.1007/s11010-023-04858-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-023-04858-1