Abstract
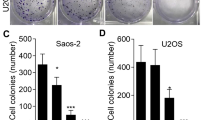
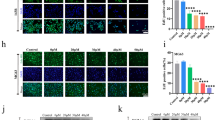
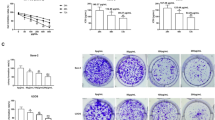
The major reason responsible for the poor prognosis of osteosarcoma is the malignant proliferation of osteosarcoma cells. The activated Wnt/β-catenin signaling induces c-MYC gene transcription and results in osteocytes’ carcinomatous change, which contributes to osteosarcoma development, so c-MYC gene is one of the therapeutic targets. The role of multiple botanical extracts in the expression of β-catenin’s target gene c-MYC in osteosarcoma MG-63 cells was tested by cellomics high content screening. Baicalein was identified as the most effective one which can inhibit the proliferation and promote the apoptosis of MG-63 cells in a dose-dependent manner by cell counting kit-8 test and fluorescence-activated cell sorting, respectively. This process was associated with the decreased levels of β-catenin and its target gene c-MYC, identified by q-PCR and Western blotting, respectively. When MG-63 cells were treated with both baicalein and JNK inhibitor SP600125, the apoptosis and expression of c-MYC were not significantly decreased. After the construct pcDNA3.1-BANCR (BRAF-regulated lncRNA 1) was transfected into MG-63 cells, RT-PCR, Western blotting and CCK-8 assay showed that BANCR was positively correlated with baicalein. These results indicated that baicalein inhibited osteosarcoma cell proliferation and promoted apoptosis by targeting c-MYC gene through Wnt signaling, in which JNK and BANCR were also involved as well as β-catenin, suggesting a new potential mechanism for us to better understand the inhibiting effect of baicalein on osteosarcoma.





Similar content being viewed by others
References
Raymond AK, Jaffe N (2009) Osteosarcoma multidisciplinary approach to the management from the pathologist’s perspective. Cancer Treat Res 152:63–84. doi:10.1007/978-1-4419-0284-9_4
Chou AJ, Geller DS, Gorlick R (2008) Therapy for osteosarcoma: where do we go from here? Paediatr Drugs 10:315–327
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. doi:10.1016/j.cell.2011.02.013
Luetke A, Meyers PA, Lewis I, Juergens H (2014) Osteosarcoma treatment—where do we stand? A state of the art review. Cancer Treat Rev 40:523–532. doi:10.1016/j.ctrv.2013.11.006
Nusse R, Varmus H (2012) Three decades of Wnts: a personal perspective on how a scientific field developed. EMBO J 31:2670–2684. doi:10.1038/emboj.2012.146
Taketo MM (2004) Shutting down Wnt signal-activated cancer. Nat Genet 36:320–322. doi:10.1038/ng0404-320
MacDonald BT, Tamai K, He X (2009) Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17:9–26. doi:10.1016/j.devcel.2009.06.016
Smeland S, Bruland OS, Hjorth L, Brosjo O, Bjerkehagen B, Osterlundh G, Jakobson A, Hall KS, Monge OR, Bjork O, Alvegaard TA (2011) Results of the Scandinavian Sarcoma Group XIV protocol for classical osteosarcoma: 63 patients with a minimum follow-up of 4 years. Acta Orthop 82:211–216. doi:10.3109/17453674.2011.566141
Esumi T, Makado G, Zhai H, Shimizu Y, Mitsumoto Y, Fukuyama Y (2004) Efficient synthesis and structure-activity relationship of honokiol, a neurotrophic biphenyl-type neolignan. Bioorg Med Chem Lett 14:2621–2625. doi:10.1016/j.bmcl.2004.02.067
Singh T, Katiyar SK (2013) Honokiol inhibits non-small cell lung cancer cell migration by targeting PGE(2)-mediated activation of beta-catenin signaling. PLoS One 8:e60749. doi:10.1371/journal.pone.0060749
Li R, Zhang L, Jia L, Duan Y, Li Y, Bao L, Sha N (2014) Long non-coding RNA BANCR promotes proliferation in malignant melanoma by regulating MAPK pathway activation. PLoS One 9:e100893
Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE (1988) Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell 55:619–625
Anastas JN, Moon RT (2013) WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer 13:11–26. doi:10.1038/nrc3419
Arora S, Singh S, Piazza GA, Contreras CM, Panyam J, Singh AP (2012) Honokiol: a novel natural agent for cancer prevention and therapy. Curr Mol Med 12:1244–1252
Hardt SE, Sadoshima J (2002) Glycogen synthase kinase-3beta: a novel regulator of cardiac hypertrophy and development. Circ Res 90:1055–1063
You L, Kim J, He B, Xu Z, McCormick F, Jablons DM (2006) Wnt-1 signal as a potential cancer therapeutic target. Drug News Perspect 19:27–31. doi:10.1358/dnp.2005.19.1.965871
Chen S, Guttridge DC, You Z, Zhang Z, Fribley A, Mayo MW, Kitajewski J, Wang CY (2001) Wnt-1 signaling inhibits apoptosis by activating beta-catenin/T cell factor-mediated transcription. J Cell Biol 152:87–96
Chae JI, Jeon YJ, Shim JH (2013) Downregulation of Sp1 is involved in honokiol-induced cell cycle arrest and apoptosis in human malignant pleural mesothelioma cells. Oncol Rep 29:2318–2324. doi:10.3892/or.2013.2353
Jeong JJ, Lee JH, Chang KC, Kim HJ (2012) Honokiol exerts an anticancer effect in T98G human glioblastoma cells through the induction of apoptosis and the regulation of adhesion molecules. Int J Oncol 41:1358–1364. doi:10.3892/ijo.2012.1582
Hahm ER, Arlotti JA, Marynowski SW, Singh SV (2008) Honokiol, a constituent of oriental medicinal herb magnolia officinalis, inhibits growth of PC-3 xenografts in vivo in association with apoptosis induction. Clin Cancer Res 14:1248–1257. doi:10.1158/1078-0432.CCR-07-1926
Hou W, Chen L, Yang G, Zhou H, Jiang Q, Zhong Z, Hu J, Chen X, Wang X, Yuan Y, Tang M, Wen J, Wei Y (2008) Synergistic antitumor effects of liposomal honokiol combined with adriamycin in breast cancer models. Phytother Res 22:1125–1132. doi:10.1002/ptr.2472
Kaldis P, Pagano M (2009) Wnt signaling in mitosis. Dev Cell 17:749–750. doi:10.1016/j.devcel.2009.12.001
Howe LR, Brown AM (2004) Wnt signaling and breast cancer. Cancer Biol Ther 3:36–41
Derksen PW, T** E, Meijer HP, Klok MD, Mac Gillavry HD, van Oers MH, Lokhorst HM, Bloem AC, Clevers H, Nusse R (2004) Illegitimate WNT signaling promotes proliferation of multiple myeloma cells. Proc Natl Acad Sci USA 101:6122–6127
Yu X, Wang Y, DeGraff DJ, Wills ML, Matusik RJ (2010) Wnt/β-Catenin activation promotes prostate tumor progression in a mouse model. Oncogene 30:1868–1879
Li Y, Bavarva JH, Wang Z, Guo J, Qian C, Thibodeau SN, Golemis EA, Liu W (2011) HEF1, a novel target of Wnt signaling, promotes colonic cell migration and cancer progression. Oncogene 30:2633–2643
Zhang H, Chen Z, Wang X, Huang Z, He Z, Chen Y (2013) Long non-coding RNA: a new player in cancer. J Hematol Oncol 6:1–7
J-p Li, L-h Liu, Li J, Chen Y, X-w Jiang, Y-r Ouyang, Y-q Liu, Zhong H, Li H, **ao T (2013) Microarray expression profile of long noncoding RNAs in human osteosarcoma. Biochem Biophys Res Commun 433:200–206
Flockhart RJ, Webster DE, Qu K, Mascarenhas N, Kovalski J, Kretz M, Khavari PA (2012) BRAFV600E remodels the melanocyte transcriptome and induces BANCR to regulate melanoma cell migration. Genome Res 22:1006–1014
Sun M, Liu X-H, Wang K-M, F-q Nie, Kong R, J-s Yang, **a R, Xu T-P, ** F-Y, Liu Z-J (2014) Downregulation of BRAF activated non-coding RNA is associated with poor prognosis for non-small cell lung cancer and promotes metastasis by affecting epithelial–mesenchymal transition. Mol Cancer 13:68
Jiang W, Zhang D, Xu B, Wu Z, Liu S, Zhang L, Tian Y, Han X, Tian D (2015) Long non-coding RNA BANCR promotes proliferation and migration of lung carcinoma via MAPK pathways. Biomed Pharmacother 69:90–95
**g L, Anning L (2005) Role of JNK activation in apoptosis: a double-edged sword. Cell Res 15:36–42
Stavniichuk R, Drel VR, Shevalye H, Maksimchyk Y, Kuchmerovska TM, Nadler JL, Obrosova IG (2011) Baicalein alleviates diabetic peripheral neuropathy through inhibition of oxidative–nitrosative stress and p38 MAPK activation. Exp Neurol 230:106–113
Q-m Zhou, Wang S, Zhang H, Y-y Lu, X-f Wang, Motoo Y, S-b Su (2009) The combination of baicalin and baicalein enhances apoptosis via the ERK/p38 MAPK pathway in human breast cancer cells. Acta Pharmacol Sin 30:1648–1658
Conflict of interest
The authors have no conflict of interest to report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, N., Zhang, Z. Baicalein suppresses the viability of MG-63 osteosarcoma cells through inhibiting c-MYC expression via Wnt signaling pathway. Mol Cell Biochem 405, 187–196 (2015). https://doi.org/10.1007/s11010-015-2410-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2410-6