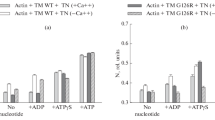
Abstract
Two single mutations, R694N and E45Q, were introduced in the beta isoform of human cardiac myosin to remove permanent salt bridges E45:R694 and E98:R694 in the SH1-SH2 helix of the myosin head. Beta isoform-specific bridges E45:R694 and E98:R694 were discovered in the molecular dynamics simulations of the alpha and beta myosin isoforms. Alpha and beta isoforms exhibit different kinetics, ADP dissociates slower from actomyosin containing beta myosin isoform, therefore, beta myosin stays strongly bound to actin longer. We hypothesize that the electrostatic interactions in the SH1-SH2 helix modulate the affinity of ADP to actomyosin, and therefore, the time of the strong actomyosin binding. Wild type and the mutants of the myosin head construct (1–843 amino acid residues) were expressed in differentiated C2C12 cells, and the duration of the strongly bound state of actomyosin was characterized using transient kinetics spectrophotometry. All myosin constructs exhibited a fast rate of ATP binding to actomyosin and a slow rate of ADP dissociation, showing that ADP release limits the time of the strongly bound state of actomyosin. The mutant R694N showed a faster rate of ADP release from actomyosin, compared to the wild type and the E45Q mutant, thus indicating that electrostatic interactions within the SH1-SH2 helix region of human cardiac myosin modulate ADP release and thus, the duration of the strongly bound state of actomyosin.










Similar content being viewed by others
References
Albet-Torres N et al (2009) Drug effect unveils inter-head cooperativity and strain-dependent ADP release in fast skeletal actomyosin. J Biol Chem 284(34):22926–22937
Batra R, Geeves MA, Manstein DJ (1999) Kinetic analysis of dictyostelium discoideum myosin motor domains with glycine-to-alanine mutations in the reactive thiol region. Biochemistry 38(19):6126–6134
Bloemink MJ, Geeves MA (2011) Shaking the myosin family tree: biochemical kinetics defines four types of myosin motor. Semin Cell Dev Biol 22(9):961–967
Cope MJTV et al (1996) Conservation within the myosin motor domain: implications for structure and function. Structure 4(8):969–987
Criddle AH, Geeves MA, Jeffries T (1985) The use of actin labeled with N-(1-Pyrenyl)Iodoacetamide to study the interaction of actin with myosin subfragments and troponin tropomyosin. Biochem J 232(2):343–349
Deacon JC et al (2012) Identification of functional differences between recombinant human alpha and beta cardiac myosin motors. Cell Mol Life Sci 69(24):4239–4255
Gargey A et al (2019) Electrostatic interactions in the force-generating region of the human cardiac myosin modulate ADP dissociation from actomyosin. Biochem Biophys Res Commun 509(4):978–982
Ge JH et al (2019) CaATP prolongs strong actomyosin binding and promotes futile myosin stroke. J Muscle Res Cell Motil 40(3–4):389–398
Hannemann DE et al (2005) Magnesium, ADP, and actin binding linkage of myosin V: evidence for multiple myosin V-ADP and actomyosin V-ADP states. Biochemistry 44(24):8826–8840
Harris DE, Warshaw DM (1993) Smooth and skeletal-muscle myosin both exhibit low duty cycles at zero load in-vitro. J Biol Chem 268(20):14764–14768
Houdusse A, Sweeney HL (2001) Myosin motors: missing structures and hidden springs. Curr Opin Struct Biol 11(2):182–194
Houk TW Jr, Ue K (1974) The measurement of actin concentration in solution: a comparison of methods. Anal Biochem 62(1):66–74
Hu AH, Wang F, Sellers JR (2002) Mutations in human nonmuscle myosin IIA found in patients with May-Hegglin anomaly and fechtner syndrome result in impaired enzymatic function. J Biol Chem 277(48):46512–46517
Iwai S, Hanamoto D, Chaen S (2006) A point mutation in the SH1 helix alters elasticity and thermal stability of myosin II. J Biol Chem 281(41):30736–30744
Koppole S, Smith JC, Fischer S (2007) The structural coupling between ATPase activation and recovery stroke in the myosin II motor. Structure 15(7):825–837
Kouyama T, Mihashi K (1981) Fluorimetry study of N-(1-Pyrenyl)Iodoacetamide-labelled F-actin - local structural-change of actin protomer both on polymerization and on binding of heavy-meromyosin. Eur J Biochem 114(1):33–38
Kushmerick MJ, Moerland TS, Wiseman RW (1992) Mammalian skeletal-muscle fibers distinguished by contents of phosphocreatine, Atp, and Pi. Proc Natl Acad Sci USA 89(16):7521–7525
Lalwani AK et al (2000) Human nonsyndromic hereditary deafness DFNA17 is due to a mutation in nonmuscle myosin MYH9. Am J Hum Genet 67(5):1121–1128
Marston SB, Taylor EW (1980) Comparison of the myosin and actomyosin atpase mechanisms of the 4 types of vertebrate muscles. J Mol Biol 139(4):573–600
Martinsson T et al (2000) Autosomal dominant myopathy: missense mutation (Glu-706 -> Lys) in the myosin heavy chain IIa gene. Proc Natl Acad Sci USA 97(26):14614–14619
Melchior NC (1954) Sodium and potassium complexes of adenosinetriphosphate: equilibrium studies. J Biol Chem 208(2):615–627
Nag S et al (2015) Contractility parameters of human beta-cardiac myosin with the hypertrophic cardiomyopathy mutation R403Q show loss of motor function. Sci Adv 1(9):e1500511
Palmiter KA et al (1999) Kinetic differences at the single molecule level account for the functional diversify of rabbit cardiac myosin isoforms. J Physiol Lond 519(3):669–678
Phillips RC, George P, Rutman RJ (1966) Thermodynamic studies of formation and ionization of magnesium(2) complexes of Adp and Atp over Ph range 5 to 9. J Am Chem Soc 88(12):2631–2631
Rosenfeld SS et al (1998) Structural and kinetic studies of phosphorylation-dependent regulation in smooth muscle myosin. J Biol Chem 273(44):28682–28690
Rosenfeld SS et al (2000) Kinetic and spectroscopic evidence for three actomyosin:ADP states in smooth muscle. J Biol Chem 275(33):25418–25426
Sasaki N, Ohkura R, Sutoh K (2003) Dictyostelium myosin II mutations that uncouple the converter swing and ATP hydrolysis cycle. Biochemistry 42(1):90–95
Segel IH (1976) Biochemical calculations. Wiley, New York
Shoup D, Lipari G, Szabo A (1981) Diffusion-controlled ligand-binding to proteins—the effect of rotational diffusion and orientation constraints. Biophys J 33(2):A308–A308
Siemankowski RF, White HD (1984) Kinetics of the interaction between actin, Adp, and cardiac myosin-S1. J Biol Chem 259(8):5045–5053
Siemankowski RF, Wiseman MO, White HD (1985) ADP dissociation from actomyosin subfragment 1 is sufficiently slow to limit the unloaded shortening velocity in vertebrate muscle. Proc Natl Acad Sci USA 82(3):658–662
Sigel H, Song B (1996) Solution studies of nucleotide-metal ion complexes—isomeric equilibria. In: Astrid Sigel HS (ed) Metal ions in biological systems. CRC Press, New York, p 135
Sleep JA, Hutton RL (1978) Actin mediated release of ATP from a myosin-ATP complex. Biochemistry 17(25):5423–5430
Sleep JA, Hutton RL (1980) Exchange between inorganic phosphate and adenosine 5’-triphosphate in the medium by actomyosin subfragment 1. Biochemistry 19(7):1276–1283
Solc K, Stockmayer WH (1971) Kinetics of diffusion-controlled reaction between chemically asymmetric molecules.1. General theory. J Chem Phys 54(7):2981-2981+
Strzelecka-Golaszewska H et al (1980) Chicken-gizzard actin: polymerization and stability. Eur J Biochem 104(1):41–52
Takagi Y et al (2008) Human myosin Vc is a low duty ratio, nonprocessive molecular motor. J Biol Chem 283(13):8527–8537
Winkelmann DA et al (2015) Structural basis for drug-induced allosteric changes to human beta-cardiac myosin motor activity. Nat Commun 6:7974
Zeng W et al (2004) Dynamics of actomyosin interactions in relation to the cross-bridge cycle. Philos Trans R Soc B 359(1452):1843–1855
Acknowledgements
This work was supported by National Institutes of Health Grant HL132315.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Ethical approval
Actin was produced from rabbit skeletal tissue. All experimental protocols were approved by the Institutional Animal Care and Use Committee of UNC Charlotte and all experiments were performed under the relevant guidelines and regulations.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gargey, A., Iragavarapu, S.B., Grdzelishvili, A.V. et al. Electrostatic interactions in the SH1-SH2 helix of human cardiac myosin modulate the time of strong actomyosin binding. J Muscle Res Cell Motil 42, 137–147 (2021). https://doi.org/10.1007/s10974-020-09588-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-020-09588-1