Abstract
Aluminium alloys are popular in modern applications due to their lightweight, high strength and ductility. Alloys in the 8xxx series have similar properties to those in the 1xxx series, but are stronger, are more malleable and have higher stiffness. The addition of rare earths (RE) can refine the as-cast microstructure and, as a result, can increase the corrosion resistance and mechanical properties of aluminium alloys at room and high temperatures. The effects of rare earth (Ce and/or La) additions to Al-1.4Fe alloys were investigated. Thermal analysis of the solidification behaviour of the reference alloy showed the occurrence of three reactions corresponding to the formation of α-Al, eutectic (α-Al + AlxFey) and Fe intermetallics, respectively. The results showed similar reactions for the Ce- and/or La-modified alloy, but at slightly different temperatures, indicating a change in the forming phases due to the addition of Ce and/or La. In all cases, the microstructures were typically hypoeutectic, consisting of the primary α-Al and the eutectic (α-Al + AlxFey). The effect of grain refinement of the primary α-Al grains of the as-cast alloy was observed by the addition of RE, while La showed the strongest effect. The effect of the RE additions showed no obvious differences in the morphology of the eutectic AlxFey, although they were present in these phases. When Ce and/or La were added, (α-Al + Al11Ce3) and/or (α-Al + Al11La3) eutectics were formed, while Fe was not detected in these eutectics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aluminium alloys are popular in modern applications due to their lightweight, high strength and ductility. Alloys in the 8xxx series have similar properties to those in the 1xxx series but have higher strength, better formability and greater stiffness [1, 2]. Incorporating rare earth (RE) into aluminium alloys presents an opportunity to enhance their microstructure, corrosion resistance and mechanical properties, at both room temperature and elevated temperatures. The practice of RE micro-alloying is a prevalent technique in conventional casting methods for diverse aluminium alloys. Additionally, it facilitates the modulation of heterostructure growth by adjusting the composition of micro-alloyed atoms in the alloy [3]. Studies have shown the positive influence of RE elements on the properties of Al–Fe aluminium alloys [4,5,6].
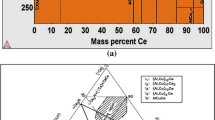
Al–Fe alloys often exhibit coarse AlxFey phases with feathery, plate-like or needle-like morphology, which can reduce ductility due to stress concentration [7]. Unfortunately, coarse AlxFey phases such as Al3Fe (Al13Fe4), AlmFe or Al6Fe usually form because the maximum solid solubility of Fe in Al is very low. To improve the strength properties of Al–Fe alloys, it is recommended to modify the microstructure using grain refiners, alloying elements or heat treatment modifications [8, 9]. AA8176 aluminium alloy, which has excellent electrical conductivity and high specific strength, has great potential for various applications [10]. Studies have shown that the addition of La-rich RE mixtures to Al–Fe alloys can refine the Al3Fe eutectic phase and improve the microstructure and mechanical properties [3]. Small quantities of La contribute to the refinement of α-Al grains [11, 12] and the alteration of the Al13Fe4 intermetallic phase, resulting in heightened elongation and electrical conductivity. Nevertheless, an excessive La may induce the formation of the Al11La3 phase, leading to a reduction in elongation [10, 12, 13].
An alternative rare earth (RE) addition to consider is Ce. As reported by Liang et al. [8], a combination of RE elements can efficiently refine both the α-Al grain and the intermetallic Al3Fe/Al6Fe in an Al-2 mass % Fe alloy. With a 0.5 mass % RE addition, the average grain size of primary α-Al becomes half the size compared to the alloy without RE. The introduction of mixed RE initially enhances and subsequently diminishes the mechanical properties of the alloys. The RE elements that accumulate before the solid–liquid interface form Al8Fe2Ce at the grain boundaries of α-Al. The addition of 0.7 mass % mixed RE resulted in preferential formation of the granular Al11Ce3 in which La was dissolved. Nonetheless, the emergence of Al11Ce3 diminished the refining influence of the mixed RE combination, adversely affecting the mechanical properties of the alloy. Through uniform annealing, the lamellar AlxFey phase is transformed into short rods, and subsequent cold rolling disperses the second phase, distributing it evenly within the primary α-Al matrix. The alloy, with a 0.5 mass % mixed RE addition, exhibits a remarkable improvement in elongation and tensile strength—50.14% and 10.67% higher, respectively—compared to the unmodified alloy in the cold-rolled state [8].
Some researchers also reported that the addition of La and Ce elements to aluminium alloys can remove harmful impurities such as Fe and Si, refine grain size, change microstructure and improve inclusion phase distribution and electrical conductivity [14]. A Ce content of 0.05–0.16 mass % in Al rods has a particularly positive effect on electrical conductivity and tensile strength, as it reduces the solid solubility of impurities in the aluminium matrix [15,16,17]. Ce exerts a potent purifying influence on Fe and Si impurities within the aluminium matrix, resulting in the creation of Al–Fe-Si-Ce compounds rather than a solid solution [Full size image