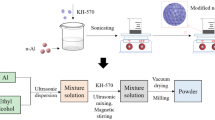
Abstract
The interfacial reaction of nano-aluminum (nAl) and fluoropolymer was evaluated using nAl@Viton composite under slow and rapid heating rates. The results under slow heating rates indicate that nAl@Viton composites reacted in several stages at 100–1000 °C. The oxide shell of nAl underwent a pre-ignition reaction with Viton before Viton decomposition, followed by the reaction of nAl with the products from Viton decomposition, and defluorination of aluminum fluoride. Temperature jump experiments were used to confirm the occurrence of interfacial reactions between nAl particles with the products produced by Viton decomposition, and the gaseous products of nAl@Viton during the reactions were investigated via online gas chromatography/mass spectrometry. Results show a direct correlation between the nAl particles and the relative abundance of products. The initial decomposition products of Viton would be induced to form more cyclic hydrocarbon by the fluorination of nAl with gaseous fluorocarbons and hydrogen fluoride released from Viton decomposition. Fluorination yields heat feedback to the reactive surface, thus providing the main advantage of aluminum–fluoropolymer reactive system for energy release.









Similar content being viewed by others
References
Glavier L, Taton G, Ducéré J, Baijot V, Pinon S, Calais T, et al. Nanoenergetics as pressure generator for nontoxic impact primers: comparison of Al/Bi2O3, Al/CuO, Al/MoO3 nanothermites and Al/PTFE. Combust Flame. 2015;162:1813–20.
McCollum J, Morey AM, Iacono ST. Morphological and combustion study of interface effects in aluminum–poly(vinylidene fluoride) composites. Mater Des. 2017;134:64–70.
Mulamba O, Pantoya ML. Exothermic surface chemistry on aluminum particles promoting reactivity. Appl Surf Sci. 2014;315:90–4.
Kappagantula KS, Farley C, Pantoya ML, Horn J. Tuning energetic material reactivity using surface functionalization of aluminum fuels. J Phys Chem C. 2012;116:24469–75.
Kappagantula KS, Pantoya ML, Horn J. Effect of surface coatings on aluminum fuel particles toward nanocomposite combustion. Surf Coat Technol. 2013;237:456–9.
Bello MN, Hill KJ, Pantoya ML, Jouet RJ, Horn JM. Surface engineered nanoparticles dispersed in kerosene: the effect of oleophobicity on droplet combustion. Combust Flame. 2018;188:243–9.
McCollum J, Pantoya ML, Iacono ST. Catalyzing aluminum particle reactivity with a fluorine oligomer surface coating for energy generating applications. J Fluorine Chem. 2015;180:265–71.
Pantoya ML, Dean SW. The influence of alumina passivation on nano-Al/Teflon reactions. Thermochim Acta. 2009;493:109–10.
Clayton NA, Kappagantula KS, Pantoya ML, Kettwich SC, Iacono ST. Fabrication, characterization, and energetic properties of metallized fibers. ACS Appl Mater Interfaces. 2014;6:6049–53.
McCollum J, Pantoya ML, Iacono ST. Activating aluminum reactivity with fluoropolymer coatings for improved energetic composite combustion. ACS Appl Mater Interfaces. 2015;7:18742–9.
Ke X, Guo S, Zhang G, Zhou X, **ao L, Hao G, et al. Safe preparation, energetic performance and reaction mechanism of corrosion-resistant Al/PVDF nanocomposite films. J Mater Chem A. 2018;6:17713–23.
Wang H, Shen J, Kline DJ, Eckman N, Agrawal NR, Wu T, et al. Direct writing of a 90 wt% particle loading nanothermite. Adv Mater. 2019;31:e1806575.
Wang H, Kline DJ, Rehwoldt M, Wu T, Zhao W, Wang X, et al. Architecture can significantly alter the energy release rate from nanocomposite energetics. ACS Appl Polym Mater. 2019;1:982–9.
Bencomo JA, Iacono ST, McCollum J. 3D printing multifunctional fluorinated nanocomposites tuning electroactivity, rheology and chemical reactivity. J Mater Chem A. 2018;6:12308–15.
He W, Liu P, Gong F, Tao B, Gu J, Yang Z, et al. Tuning the reactivity of metastable intermixed composite n-Al/PTFE by polydopamine interfacial control. ACS Appl Mater Inter. 2018;10:32849–58.
Row SL, Groven LJ. Smart energetics: sensitization of the aluminum–fluoropolymer reactive system. Adv Eng Mater. 2018;20:1700409.
Crouse CA, Pierce CJ, Spowart JE. Synthesis and reactivity of aluminized fluorinated acrylic (AlFA) nanocomposites. Combust Flame. 2012;159:3199–207.
Valluri SK, Schoenitz M, Dreizin E. Fluorine-containing oxidizers for metal fuels in energetic formulations. Def Technol. 2019;15:1–22.
DeLisio JB, Hu X, Wu T, Egan GC, Young G, Zachariah MR. Probing the reaction mechanism of aluminum/poly(vinylidene fluoride) composites. J Phys Chem B. 2016;120:5534–42.
Yan T, Ren H, Li Y, Wang H, Jiao Q. Tailoring structural energetics for enhanced reactivity of nano-aluminum particles based microspheres. Adv Eng Mater. 2019;21:1900176.
Cadman P, Gossedge GM. The chemical interaction of metals with polytetrafluoroethylene. J Mater Sci. 1979;14:2672–8.
Osborne DT, Pantoya ML. Effect of Al particle size on the thermal degradation of Al/Teflon mixtures. Combust Sci Technol. 2007;179:1467–80.
Noor F, Zhang H, Korakianitis T, Wen D. Oxidation and ignition of aluminum nanomaterials. Phys Chem Chem Phys. 2013;15:20176–88.
Trunov MA, Schoenitz M, Zhu X, Dreizin EL. Effect of polymorphic phase transformations in Al2O3 film on oxidation kinetics of aluminum powders. Combust Flame. 2005;140:310–8.
Sarbak Z. Effect of fluoride and sodium ions on structural and thermal properties of γ-Al2O3. Cryst Res Technol. 1997;32:491–7.
Deshmukh SS, Kovalchuk VI, Borovkov VY, D’Itri JL. FTIR spectroscopic and reaction kinetics study of the interaction of CF3CFCl2 with γ-Al2O3. J Phys Chem B. 2000;104:1277–84.
Riello D, Zetterström C, Parr C, Braulio MAL, Moreira M, Gallo JB, et al. AlF3 reaction mechanism and its influence on α-Al2O3 mineralization. Ceram Int. 2016;42:9804–14.
Lonfei J, **gling W, Shuman X. Mechanisms of pyrolysis of fluoropolymers. J Anal Appl Pyrol. 1986;10:99–106.
Watson KW, Pantoya ML, Levitas VI. Fast reactions with nano- and micrometer aluminum: a study on oxidation versus fluorination. Combust Flame. 2008;155:619–34.
Choi S, Kim Y. Analysis of pyrolysis products of poly(vinylidene fluoride-co-hexafluoropropylene) by pyrolysis-gas chromatography/mass spectrometry. J Fluor Chem. 2014;165:33–8.
Choi S, Kim Y. Microstructural analysis of poly(vinylidene fluoride) using benzene derivative pyrolysis products. J Anal Appl Pyrol. 2012;96:16–23.
Losada M, Chaudhuri S. Theoretical study of elementary steps in the reactions between Aluminum and Teflon fragments under combustive environments. J Phys Chem A. 2009;113:5933–41.
Shin T, Hajime O, Chuichi W. Pyrolysis-GC/MS data book of synthetic polymers: pyrograms, thermograms and MS of pyrolyzates. Amsterdam: Elsevier B.V; 2012.
Hiltz JA. Characterization of fluoroelastomers by various analytical techniques including pyrolysis gas chromatography/mass spectrometry. J Anal Appl Pyrol. 2014;109:283–95.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 11832006, U1530262).
Author information
Authors and Affiliations
Contributions
TY contributed to conceptualization, methodology, formal analysis, writing—original draft; HR contributed to project administration, investigation; QC contributed to conceptualization, writing—original draft, writing—review and editing; YO contributed to methodology, formal analysis; FG contributed to data curation.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yan, T., Ren, H., Cui, Q. et al. What happens to the interfacial reaction between fluoropolymer and nano aluminum below 1000 ℃?. J Therm Anal Calorim 147, 8657–8666 (2022). https://doi.org/10.1007/s10973-021-11101-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-11101-w