Abstract
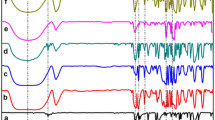
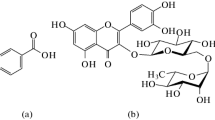
In this study, an inclusion complex of benzoic acid with β-cyclodextrin (BA-βCD) was obtained from water–ethanol solvents. The yield of complex synthesis in binary mixtures is greater than in water and reaches maximum value at 0.10 mol fraction of ethanol. Results of FTIR spectroscopy analysis showed that the main difference in the spectra of the acid and inclusion complex was observed in the frequency ranging from 2500 to 3100 cm−1, corresponding to aromatic hydrogen vibrations. These vibrations are highly attenuated in complex. Phase solubility and differential scanning calorimetry studies revealed that the inclusion complex was obtained with 1:1 stoichiometric ratio and the solubility of benzoic acid increased with an increase in β-cyclodextrin concentrations in water. The logarithm of stability constant in water was found to be lgK = 1.99. The thermodynamic parameters for the reaction of (BA-βCD) complex formation in H2O–EtOH solvents were determined from calorimetric experiments carried out by means of the calorimetric titration system TAM III (TA Instruments) at T = 25 °C. The heat effects of mixing β-cyclodextrin solutions with benzoic acid were obtained from water–ethanol mixed solvents containing X(EtOH) = (0.00, 0.10, 0.20 and 0.30) mole fraction at pH = 3.6 and T = 25 °C. However, at X(EtOH) = 0.30 mol fraction, according to the calorimetric titration data, no complex formation occurs. When transferring from H2O to H2O–EtOH solvents, complex stability decreases from lgK = 2.4 to lgK = 0.7, wherein the reaction exothermicity increases from − 12.2 kJ mol−1 to − 44.3 kJ mol−1. An increase in the exothermicity of complexation is accompanied by a decrease in the entropic contribution to the change in the reaction Gibbs energy.






Similar content being viewed by others
References
Mendez SG, Espinar FJ, Alvarez AL, Longhi MR, Quevedo MA, Zoppi A. Ternary complexation of benzoic acid with β-cyclodextrin and aminoacids. Experimental and theoretical studies. J Incl Phenom Macrocycl Chem. 2016;85:33–48.
Terekhova I, Koz’biał M, Kumeev R, Gierycz P. Complex formation of native and hydroxypropylated cyclodextrins with benzoic acid in aqueous solution: volumetric and 1H NMR study. Chem Phys Lett. 2011;514:341–6.
Brewster ME, Vandecruys R, Peeters J, Neeskens P, Verreck G, Loftsson T. Comparative interaction of 2-hydroxypropyl-beta-cyclodextrin and sulfobutylether-beta-cyclodextrin with itraconazole: phase-solubility behavior and stabilization of supersaturated drug solutions. Eur J Pharm Sci. 2008;34(2–3):94–103.
Yan H, ** L, Samuel HY. Solubilization of Fluasterone in cosolvent/cyclodextrin combinations. Int J Pharm. 2003;264:25–34.
Soares-Sobrinho JL, Santos FL, Lyra MA, Alves LD, Rolim LA, Lima AA, Nunes LC, Soares MF, Rolim-Neto PJ, Torres-Labandeira JJ. Benznidazole drug delivery by binary and multicomponent inclusion complexes using cyclodextrins and polymers. Carbohydr Polym. 2012;89(2):323–30.
Challa R, Ahuja A, Ali J, Khar RK. Cyclodextrins in drug delivery: an updated review. AAPS Pharm Sci Tech. 2005;6:329–57.
Kustov AV, Smirnova NL, Neueder R, Kunz W. Amino acid solvation in aqueous kosmotrope solutions: temperature dependence of the L-Histidine-Glycerol interaction. J Phys Chem B. 2012;116:2325–9.
Kustov AV. The aromatic amino acid behaviour in aqueous amide solutions—the temperature dependence of the L-phenylalanine-urea interaction. J Thermal Anal Calorim. 2007;89:841–6.
Donze C, Coleman AW. Solvent effects in competition between guest molecules for β-cyclodextrin. J Incl Phenom Mol Recogn Chem. 1995;23:11–21.
Yoshii H, Kometani T, Furuta T, Watanabe Y, Linko YY. Formation of inclusion complexes of cycldextrin with ethanol under anhydrous conditions. Biosci Biotechnol Biochem. 1998;62:2166–70.
He Y, Li P, Yalkowsky SH. Solubilization of Fluasterone in cosolvent/cyclodextrin combinations. Inter J Pharm. 2003;264:25–34.
Zung JB, Munoz de la Pena A, Ndou TT, Warner IM. Influence of alcohol addition on the γ-CD: pyrene complex. J Phys Chem. 1991;95:6701–9.
Reer O, Muller BW. Investigation of the influence of cosolvents and surfactants on the complexation of dexamethasone with hydroxypropyl-β-cyclodextrin by use of a simplex lattice design. Eur J Pharm Biopharm. 1993;39:105–11.
Loftsson T, Ólafsdóttir BJ, Friðriksdóttir H, Jónsdóttir S. Cyclodextrin complexation of NSAIDs: physicochemical characteristics. Eur J Pharm Sci. 1993;1:95–101.
Pitha J, Hoshino T. Effect of ethanol on formation of inclusion complexes of hydroxypropylcyclodextrins with testosterone or with methyl orange. Inter J Pharm. 1992;80:243–51.
Belyakova A, Varvarin AM, Khora OV, Oranskaya EI. The Interaction of β-Cyclodextrin with benzoic acid. Russ J Phys Chem A. 2008;82:228–32.
Terekhova IV. Comparative thermodynamic study on complex formation of native and hydroxypropylated cyclodextrins with benzoic acid. Therm Acta. 2011;526:118–21.
Shimkin A. Optimization of DSC calibration procedure. Thermochim Acta. 2013;566:71–6.
Eysel W, Breuer KH. The calorimetric calibration of differential scanning calorimetry cells. Thermochim Acta. 1982;57:317–29.
Higuchi T, Connors KA. Phase-solubility techniques. Adv Anal Chem Instrum. 1965;4:117–2122.
Wadso I, Goldberg RN. Standard in isothermal microcalorimetry. Pure Appl Chem. 2001;73:1625–39.
Usacheva TR, Sharnin VA, Chernov IV, Matteoli E. Calorimetric investigation of the reaction of molecular complex formation of 18-crown-6 with D, L-alanine in water–ethanol mixtures. J Therm Anal Calorim. 2013;112:983–9.
Usacheva TR, Pham Thi L, Kuzmina KI, Sharnin VA. Thermodynamics of complex formation between Cu(II) and glycyl–glycyl–glycine in water–ethanol and water–dimethylsulfoxide solvent. J Therm Anal Calorim. 2017;130:471–8.
Usacheva TR, Pham Thi L, Terekhova IV, Kumeev RS, Sharnin VA. Application of isothermal titration calorimetry for evaluation of water-acetone and water-dimethylsulfoxide solvents influence on the molecular complex formation between 18-crown-6 and triglycine at 298.15K. J Therm Anal Calorim. 2015;121:975–81.
Usacheva TR, Pham Thi L, Sharnin VA. Calorimetric study of the molecular complex formation of glycyl–glycyl–glycine with 18-crown-6 in aqueous organic solvents. Russ J Gen Chem. 2017;87:591–9.
Chatjigakis AK, Donze C, Coleman AW. Solubility Behavior of β-Cyclodextrin In Water/cosolvent Mixtures. Anal Chem. 1992;64:1632–4.
Coleman AW, Munoz M, Chatjigakis AK. Classification of the solubility behaviour of β-cyclodextrin in aqueous-co-solvent mixtures. J Phys Org Chem. 1993;6:651–9.
Meshkov AN, Gamov GA. KEV: a free software for calculating the equilibrium composition and determining the equilibrium constants using UV–Vis and potentiometric data. Talanta. 2019;198:200–5.
**ngen H, Ruisen L, Hanxing Z. Enthalpies and entropies of dissolution and dissociation of benzoic acid in EtOH-H2O and i-PrOH-EtOH-H2O mixtures. Acta Phys Chim Sinica. 1999;15:838–44.
Usacheva TR, Ledenkov SF, Sharnin VA. Complex formation of Ag+ with polyether 18-crown-6. Calorimetric and potentiometric methods. J Therm Anal Calorim. 2002;70:379–85.
Arnett EM, Bentrude WG, Burke JJ, MccDuggleby P. Solvent effects in organic chemistry. V. Molecules, ions, and transition states in aqueous–ethanol. J Amer Chem Soc. 1965;87:1541–52.
Afanasiev VN, Efremova LS, Volkova TV. Physical-chemical properties of binary solvents. Water-containing systems; Publisher: Academy of Sciences of the Union of Soviet Republics (USSR) Institute of non-aqueous solution chemistry. Russia. 1988; Part I, p. 104 (in Russian).
Szejtli J. Introduction and general overview of cyclodextrin chemistry. Chem Rev. 1998;98:1743–53.
Sifaoui H, Modarressi A, Magri P, Stachowicz-Kuśnierz A, Korchowiec J, Rogalski M. Formation of β-cyclodextrin complexes in an anhydrous environment. J Mol Model. 2016;22:207–20.
Price DM. Temperature calibration of differential scanning calorimeters. J Therm Anal. 1995;45:1285–96.
Sorum CH, Durand EA. The melting of binary eutectics. J Am Chem Soc. 1952;74:1071–3.
Murray JP, Cavell KJ, Hill JO. A DSC study of benzoic acid. A suggested calibrant compound. Thermochim Acta. 1980;36:97–101.
Kim KH, Frank MJ, Henderson NL. Application of differential scanning calorimetry to the study of solid drug dispersions. J Pharm Sci. 1985;74:283–9.
Borodin VA, Vasilev VP, Kozlovskiy EA. Processing of results of calorimetric measurements on a computer when studying the complex equilibria in solutions. Zh Neorg Khim. 1982;27:2169–72 (in Russian).
Harata K. Induced circular dichroism of cycloamylose complexes with meta-and para-disubstituted benzenes. Bioorg Chem. 1981;10:255–65.
Lewis EA, Hansen LD. Thermodynamics of binding of guest molecules to α and β-cyclodextrins. J Chem Soc Perkin Trans. 1972;2:2081–5.
Krestov GA. Ionic solvation, Ellis Horwood Ed., New York-London-Toronto-Sydney-Tokyo-Singapore, 1994.
Belica S, Sadowska M, Stepniak A, Graca A, Pałecz B. Enthalpy of solution of α- and β-cyclodextrin in water and in some organic solvents. J Chem Therm. 2014;69:112–7.
Usacheva TR, Sharnin VA. A thermodynamic study of reactions of amino acids with crown ethers in nonaqueous media as examples of guest-host molecular complex formation. Russ Chem Bull. 2015;64:2536–44.
Usacheva TR, Kabirov DN, Beregova DA, Gamov GA, Sharnin VA, Marco B, Laura M, Concetta G. Thermodynamics of complex formation between hydroxypropyl-β-cyclodextrin and quercetin in water-ethanol solvents at T = 298.15 K. J Therm Anal Calorim. 2019;138:417–24.
Acknowledgements
This work was funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under the Grant Number 104.06–2017.329 by RFBR and VAST according to the research project No. 19–53-54004 and by Ministry of Foreign Affairs and International Cooperation of Italy [grants in favor of foreign citizens not residing in Italy and Italian citizens living abroad, No. 946–22/10/2018]. ITC measurements presented in this work were carried out at the Institute of Thermodynamics and Kinetics of Chemical Processes of the Ivanovo State University of Chemistry and Technology (ISUCT) using the equipment of the Center for Collective Use of ISUCT. The authors thank the University of Naples Federico II for the financial support of their collaboration which contributed to the preparation of this paper.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Usacheva, T., Pham, T.L., Nguyen, T.D. et al. Host–guest inclusion complex of β-cyclodextrin and benzoic acid in water–ethanol solvents: spectroscopic and thermodynamic characterization of complex formation. J Therm Anal Calorim 142, 2015–2024 (2020). https://doi.org/10.1007/s10973-020-09807-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-09807-4