Abstract
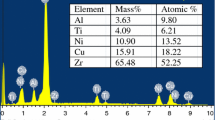
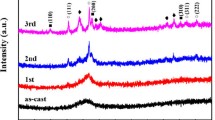
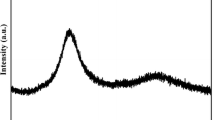
The crystallization transformation kinetics of Ti20Zr20Hf20Be20(Cu50Ni50)20 high-entropy bulk metallic glass under non-isothermal conditions are investigated using differential scanning calorimetry. The alloy shows two distinct crystallization events. The activation energies of the crystallization events are determined using Kissinger, Ozawa and Augis–Bennett methodologies. Further, we observe that similar values are obtained using the three equations. The activation energy of the initial crystallization event is observed to be slightly small as compared to that of the second event. This implies that the initial crystallization event may have been easier to be occurred. The local activation energy (E(x)) maximizes in the initial stage of crystallization and keeps drop** in subsequent crystallization process. The non-isothermal crystallization kinetics are further analyzed using the modified Johnson–Mehl–Avrami (JMA) equation. Further, the Avrami exponent values are observed to be 1.5 < n(x) < 2.5 for approximately the entire period of the initial crystallization event and for most instances (0.1 < x < 0.6) of the second crystallization event, which implies that the mechanism of crystallization is significantly controlled by diffusion-controlled two- and three-dimensional growth along with a decreasing nucleation rate.











Similar content being viewed by others
References
Yeh JW, Chen SK, Lin SJ, Gan JY, Chin TS, Shun TT, Tsau CH. Nanostructured high-entropy alloys with multiple principal elements: novel alloy design concepts and outcomes. Adv Eng Mater. 2004;6:299–303.
Zhang Y, Zhou YJ, Lin JP, Chen GL, Liaw PK. Solid-solution phase formation rules for multi-component alloys. Adv Eng Mater. 2008;10:534–8.
Zhang Y, Yang X, Liaw PK. Alloy design and properties optimization of high-entropy alloys. JOM. 2012;64:830–8.
Zhang Y, Zuo T, Tang Z, Gao MC, Dahmen KA, Liaw PK. Microstructures and properties of high-entropy alloys. Prog Mater Sci. 2014;61:1–93.
Zhou YJ, Zhang Y, Wang YL, Chen GL. Microstructure and compressive properties of multicomponent Alx(TiVCrMnFeCoNiCu)(100−x) high-entropy alloys. Mater Sci Eng A. 2007;454–455:260–5.
Otto F, Yang Y, Bei H, George EP. Relative effects of enthalpy and entropy on the phase stability of equiatomic high-entropy alloys. Acta Mater. 2013;61:2628–38.
Li HF, **e XH, Zhao K, Wang YB, Zheng YF, Wang WH, Qin L. In vitro and in vivo studies on biodegradable CaMgZnSrYb high-entropy bulk metallic glass. Acta Biomater. 2013;9:8561–73.
Zhang Y, Zuo TT, Gao ZT, Gao MC, Dahmen KA, Liaw KA, Lu ZP. Microstructures and properties of high-entropy alloys. Prog Mater Sci. 2014;61:1–93.
Ding HY, Shao Y, Gong P, Li JF, Yao KF. A senary TiZrHfCuNiBe high entropy bulk metallic glass with large glass-forming ability. Mater Lett. 2014;125:151–3.
Gao XQ, Zhao K, Ke HB, Ding DW, Wang WH, Bai HY, et al. High mixing entropy bulk metallic glasses. J Non Cryst Solids. 2011;357:3557–60.
Tong Y, Qiao JC, Zhang C, Pelletier JM, Yao Y. Mechanical properties of Ti16.7Zr16.7Hf16.7Cu16.7Ni16.7Be16.7 high-entropy bulk metallic glass. J Non Cryst Solids. 2016;452:57–61.
Zhao SF, Shao Y, Liu X, Chen N, Ding HY, Yao KF. Pseudo-quinary Ti20Zr20Hf20Be20(Cu20−xNix) high entropy bulk metallic glasses with large glass forming ability. Mater Des. 2015;87:625–31.
Zhao SF, Yang GN, Ding HY, Yao KF. A quinary Ti–Zr–Hf–Be–Cu high entropy bulk metallic glass with a critical size of 12 mm. Intermetallics. 2015;61:47–50.
Ding HY, Yao KF. High entropy Ti20Zr20Cu20Ni20Be20 bulk metallic glass. J Non Cryst Solids. 2013;364:9–12.
Takeuchi A, Chen N, Wada T, Yokoyama Y, Kato H, Inoue A, et al. Pd20Pt20Cu20Ni20P20 high-entropy alloy as a bulk metallic glass in the centimeter. Intermetallics. 2011;19:1546–54.
Gong P, Yao KF, Ding HY. Crystallization kinetics of TiZrHfCuNiBe high entropy bulk metallic glass. Mater Lett. 2015;156:146–9.
Li B, Li Yh, Yang K, Li JS, Fan XH. Effect of yttrium addition on the non-isothermal crystallization kinetics and fragility of Cu–Zr–Al bulk metallic glass. Thermochim Acta. 2016;642:105–10.
Cui J, Li JS, Wang J, Kou HC, Qiao JC, Gravierc S, Blandinc JJ. Crystallization kinetics of Cu38Zr46Ag8Al8 bulk metallic glass in different heating conditions. J Non Cryst Solids. 2014;404:7–12.
Hu XX, Jichao Q, Pelletier JM, Yao Y. Evaluation of thermal stability and isochronal crystallization kinetics in the Ti40Zr25Ni8Cu9Be18 bulk metallic glass. J Non Cryst Solids. 2016;432:254–64.
Cao QP, Liu JW, Li JF, Zhou YH, Wang XD, Jiang JZ. Isochronal crystallization kinetics of Cu60Zr20Ti20 bulk metallic glass. J Non Cryst Solids. 2011;357:1182–7.
Wang XF, Wang D, Zhu B, Li YJ, Han FS. Crystallization kinetics and thermal stability of mechanically alloyed Al76Ni8Ti8Zr4Y4 glassy powder. J Non Cryst Solids. 2014;385:111–6.
Kong LH, Gao YL, Song TT, Wang G, Zhai QJ. Non-isothermal crystallization kinetics of FeZrB amorphous alloy. Thermochim Acta. 2011;522:166–72.
Wang HR, Gao YL, Min GH, Hui XD, Ye YF. Primary crystallization in rapidly solidified Zr70Cu20Ni10 alloy from a supercooled liquid region. Phys Lett A. 2003;314:81–7.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Ozawa T. Kinetic analysis of derivative curves in thermal analysis. J Therm Anal Calorim. 1970;2:301–24.
Augis JA, Bennett JE. Calculation of the Avrami parameters for heterogeneous solid state reactions using a modification of the Kissinger method. J Therm Anal Calorim. 1978;13:283–99.
Hu XX, Jichao Q, Pelletier JM, Yao Y. Evaluation of thermal stability and isochronal crystallization kinetics in the Ti40Zr25Ni8Cu9Be18 bulk metallic glass. J Non Cryst Solids. 2016;432:254–64.
Prajapati SR, Sauthor K, Ashmi TP, Pratap A. Non-isothermal crystallization kinetics of Zr52Cu18Ni14Al10Ti6 metallic glass. J Therm Anal Calorim. 2016;124:21–33.
Cao QP, Liu JW, Li JF, Zhou YH, Wang XD, Jiang JZ. Isochronal crystallization kinetics of Cu60Zr20Ti20 bulk metallic glass. J Non Cryst Solids. 2011;357:1182–7.
Johnson WA, Mehl RF. Reaction kinetics in processes of nucleation and growth. Trans AIME. 1939;135:416–58.
Wang J, Kou HC, Li JS, Gu XF, Zhong H, Chang H, Zhou L. An integral fitting method for analyzing the isochronal transformation kinetics: application to the crystallization of a Ti-based amorphous alloy. J Phys Chem Solids. 2009;70:1448–53.
Blázquez JS, Conde CF, Conde A. Non-isothermal approach to isokinetic crystallization processes: application to the nanocrystallization of HITPERM alloys. Acta Mater. 2005;53:2305–11.
Song KK, Gargarella P, Pauly S, Ma GZ, Kuhn U, Eckert J. Correlation between glass-forming ability, thermal stability, and crystallization kinetics of Cu–Zr–Ag metallic glasses. J Appl Phys. 2012;112:063503.
Lesz S, Kwapuliński P, Nabiałek M, Zackiewicz P, Hawelek L. Thermal stability, crystallization and magnetic properties of Fe–Co-based metallic glasses. J Therm Anal Calorim. 2016;125:1143–9.
Cheng S, Wang C, Ma M, Shan D, Guo B. Non-isothermal crystallization kinetics of Zr41.2Ti13.8Cu12.5Ni10Be22.5 amorphous alloy. Thermochim Acta. 2014;587:11–7.
Zhuang YX, Duan TF, Shi HY. Calorimetric study of non-isothermal crystallization kinetics of Zr60Cu20Al10Ni10 bulk metallic glass. J Alloys Compd. 2011;509:9019–25.
Zhang LC, Xu J, Eckert J. Thermal stability and crystallization kinetics of mechanically alloyed TiC/Ti-based metallic glass matrix composite. J Appl Phys. 2006;100:033514.
Cao QP, Li JW, Li JF, Zhou YH, Wang XD, Jiang JZ. Isochronal crystallization kinetics of Cu60Zr20Ti20 bulk metallic glass. J Non Cryst Solids. 2011;357:1182–7.
Ranganathan S, Heimendahl MV. The three activation energies with isothermal transformations: applications to metallic glasses. J Mater Sci. 1981;16:2401–4.
Acknowledgements
The research was financially supported by the Special Research Project for the Education Department of Shaanxi Province (Grant No. 14JK1351) and the President fund of **’an Technological University (Grant No. 0852-302021407).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yang, K., Fan, X., Li, B. et al. Non-isothermal crystallization kinetics of Ti20Zr20Hf20Be20(Cu50Ni50)20 high-entropy bulk metallic glass. J Therm Anal Calorim 132, 979–988 (2018). https://doi.org/10.1007/s10973-018-6957-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-6957-9