Abstract
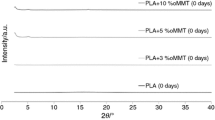
The influence of neat and organically modified montmorillonite on the structure–property relationships of a β-nucleated polypropylene matrix has been thoroughly investigated. High-angle annular dark field scanning transmission electron microscopy revealed that the organic modification of clay facilitated the dispersion of the clay, while X-ray diffractograms showed the α-nucleating effect of the clays on the β-nucleated matrix. The results from tensile tests showed that the organic modification of MMT affected profoundly only the tensile strength at yield and at break. The effect of the organic modification of the clay on the thermal stability of the composites was finally evaluated by thermogravimetric analysis, where the samples filled with oMMT decomposed faster than the ones filled with neat MMT, due to the decomposition of the organic salts that were initially used for the modification of MMT. A kinetics study of the thermal degradation of the composites was also performed, in order to export additional conclusions on the activation energy of the samples.












Similar content being viewed by others
References
Usuki A, Kojima Y, Kawasumi M, Okada A, Fukushima Y, Kurauchi T, et al. Synthesis of nylon 6-clay hybrid. J Mater Res. 1993;8(5):1179–84.
Kojima Y, Usuki A, Kawasumi M, Okada A, Fukushima Y, Kurauchi T, et al. Mechanical properties of nylon 6-clay hybrid. J Mater Res. 1993;8(5):1185–9.
Giannelis EP. Polymer layered silicate nanocomposites. Adv Mater. 1996;8(1):29–35.
LeBaron PC, Wang Z, Pinnavaia TJ. Polymer-layered silicate nanocomposites: an overview. Appl Clay Sci. 1999;15(1):11–29.
Sinha Ray S, Okamoto M. Polymer/layered silicate nanocomposites: a review from preparation to processing. Prog Polym Sci. 2003;28(11):1539–641.
Krishnamoorti R, Vaia RA, Giannelis EP. Structure and dynamics of polymer-layered silicate nanocomposites. Chem Mater. 1996;8(8):1728–34.
Kornmann X, Lindberg H, Berglund LA. Synthesis of epoxy–clay nanocomposites: influence of the nature of the clay on structure. Polymer. 2001;42(4):1303–10.
Liu X, Wu Q, Berglund LA, Fan J, Qi Z. Polyamide 6-clay nanocomposites/polypropylene-grafted-maleic anhydride alloys. Polymer. 2001;42(19):8235–9.
Shelley J, Mather P, DeVries K. Reinforcement and environmental degradation of nylon-6/clay nanocomposites. Polymer. 2001;42(13):5849–58.
Brune DA, Bicerano J. Micromechanics of nanocomposites: comparison of tensile and compressive elastic moduli, and prediction of effects of incomplete exfoliation and imperfect alignment on modulus. Polymer. 2002;43(2):369–87. doi:10.1016/S0032-3861(01)00543-2.
Masenelli-Varlot K, Reynaud E, Vigier G, Varlet J. Mechanical properties of clay-reinforced polyamide. J Polym Sci, Part B: Polym Phys. 2002;40(3):272–83.
Sheng N, Boyce MC, Parks DM, Rutledge G, Abes J, Cohen R. Multiscale micromechanical modeling of polymer/clay nanocomposites and the effective clay particle. Polymer. 2004;45(2):487–506.
Antoniadis G, Paraskevopoulos KM, Vassiliou AA, Papageorgiou GZ, Bikiaris D, Chrissafis K. Nonisothermal melt-crystallization kinetics for in situ prepared poly(ethylene terephthalate)/monmorilonite (PET/OMMT). Thermochim Acta. 2011;521(1–2):161–9. doi:10.1016/j.tca.2011.04.019.
Vassiliou AA, Chrissafis K, Bikiaris DN. In situ prepared PET nanocomposites: effect of organically modified montmorillonite and fumed silica nanoparticles on PET physical properties and thermal degradation kinetics. Thermochim Acta. 2010;500(1–2):21–9. doi:10.1016/j.tca.2009.12.005.
Maria HJ, Lyczko N, Nzihou A, Joseph K, Mathew C, Thomas S. Stress relaxation behavior of organically modified montmorillonite filled natural rubber/nitrile rubber nanocomposites. Appl Clay Sci. 2014;87:120–8. doi:10.1016/j.clay.2013.10.019.
Mejía A, García N, Guzmán J, Tiemblo P. Surface modification of sepiolite nanofibers with PEG based compounds to prepare polymer electrolytes. Appl Clay Sci. 2014;95:265–74. doi:10.1016/j.clay.2014.04.023.
Yin H, Ma L, Gan M, Li Z, Shen X, **e S, et al. Preparation and properties of poly(2,3-dimethylaniline)/organic–kaolinite nanocomposites via in situ intercalative polymerization. Compos Sci Technol. 2014;94:139–46. doi:10.1016/j.compscitech.2014.01.021.
Liu P, Zhu L, Guo J, Wang A, Zhao Y, Wang Z. Palygorskite/polystyrene nanocomposites via facile in situ bulk polymerization: gelation and thermal properties. Appl Clay Sci. 2014;. doi:10.1016/j.clay.2014.06.023.
Greesh N, Sanderson R, Hartmann PC. Functionalization of montmorillonite by end-chain mono-cationic polystyrene and end-chain mono-cationic poly(styrene-b-2-hydroxethyl acrylate). Appl Clay Sci. 2014;93–94:38–47. doi:10.1016/j.clay.2013.12.028.
Neppalli R, Causin V, Marega C, Modesti M, Adhikari R, Scholtyssek S, et al. The effect of different clays on the structure, morphology and degradation behavior of poly(lactic acid). Appl Clay Sci. 2014;87:278–84. doi:10.1016/j.clay.2013.11.029.
Varga J. β-modification of isotactic polypropylene: preparation, structure, processing, properties, and application. J Macromol Sci, Phys. 2002;41 B(4–6):1121–71.
Menyhárd A, Varga J, Molnár G. Comparison of different-nucleators for isotactic polypropylene, characterisation by DSC and temperature-modulated DSC (TMDSC) measurements. J Therm Anal Calorim. 2006;83(3):625–30.
Jones AT, Aizlewood JM, Beckett DR. Crystalline forms of isotactic polypropylene. Die Makromolekulare Chemie. 1964;75(1):134–58. doi:10.1002/macp.1964.020750113.
Varga J, Mudra I, Ehrenstein GW. Crystallization and melting of β-nucleated isotactic polypropylene. J Therm Anal Calorim. 1999;56(3):1047–57.
Menyhárd A, Dora G, Horváth Z, Faludi G, Varga J. Kinetics of competitive crystallization of β- and α-modifications in β-nucleated iPP studied by isothermal stepwise crystallization technique. J Therm Anal Calorim. 2012;108(2):613–20.
Chen HB, Karger-Kocsis J, Wu JS, Varga J. Fracture toughness of α- and β-phase polypropylene homopolymers and random- and block-copolymers. Polymer. 2002;43(24):6505–14. doi:10.1016/S0032-3861(02)00590-6.
Bárány T, Izer A, Karger-Kocsis J. Impact resistance of all-polypropylene composites composed of alpha and beta modifications. Polym Test. 2009;28(2):176–82.
Papageorgiou DG, Bikiaris DN, Chrissafis K. Effect of crystalline structure of polypropylene random copolymers on mechanical properties and thermal degradation kinetics. Thermochim Acta. 2012;543:288–94.
Naffakh M, Díez-Pascual AM, Marco C, Ellis G. Novel polypropylene/inorganic fullerene-like WS2 nanocomposites containing a β-nucleating agent: mechanical, tribological and rheological properties. Mater Chem Phys. 2014;144(1–2):98–106. doi:10.1016/j.matchemphys.2013.12.020.
Bao R-Y, Cao J, Liu Z-Y, Yang W, **e B-H, Yang M-B. Towards balanced strength and toughness improvement of isotactic polypropylene nanocomposites by surface functionalized graphene oxide. J Mater Chem A. 2014;2(9):3190–9. doi:10.1039/c3ta14554a.
Dai X, Zhang Z, Wang C, Ding Q, Jiang J, Mai K. A novel montmorillonite with β-nucleating surface for enhancing β-crystallization of isotactic polypropylene. Compos A Appl Sci Manuf. 2013;49:1–8. doi:10.1016/j.compositesa.2013.01.016.
Zhang Z, Wang C, Meng Y, Mai K. Synergistic effects of toughening of nano-CaCO3 and toughness of β-polypropylene. Compos A Appl Sci Manuf. 2012;43(1):189–97. doi:10.1016/j.compositesa.2011.10.008.
Naffakh M, Marco C, Ellis G. Novel polypropylene/inorganic fullerene-like WS2 nanocomposites containing a β-nucleating agent: isothermal crystallization and melting behavior. J Phys Chem B. 2012;116(6):1788–95.
Dou Q, Meng M-R, Li L. Effect of pimelic acid treatment on the crystallization, morphology, and mechanical properties of isotactic polypropylene/mica composites. Polym Compos. 2010;31(9):1572–84. doi:10.1002/pc.20945.
Duan J, Dou Q. Investigation on β-polypropylene/PP-g-MAH/surface treated talc composites. J Appl Polym Sci. 2013;130(1):206–21. doi:10.1002/app.39178.
Duan J, Dou Q. Investigation on β-polypropylene/PP-g-MAH/surface-treated calcium carbonate composites. Polym Compos. 2012;33(12):2245–61. doi:10.1002/pc.22370.
**e W, Gao Z, Pan WP, Hunter D, Singh A, Vaia R. Thermal degradation chemistry of alkyl quaternary ammonium Montmorillonite. Chem Mater. 2001;13(9):2979–90. doi:10.1021/cm010305s.
Bhat G, Hegde RR, Kamath M, Deshpande B. Nanoclay reinforced fibers and nonwovens. J Eng Fabr Fibers (JEFF). 2008;3(3).
Li X, Hu K, Ji M, Huang Y, Zhou G. Calcium dicarboxylates nucleation of β-polypropylene. J Appl Polym Sci. 2002;86(3):633–8.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520(1):1–19.
Vyazovkin S, Chrissafis K, Di Lorenzo ML, Koga N, Pijolat M, Roduit B, et al. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim Acta. 2014;590:1–23. doi:10.1016/j.tca.2014.05.036.
Chiu FC, Lai SM, Chen JW, Chu PH. Combined effects of clay modifications and compatibilizers on the formation and physical properties of melt-mixed polypropylene/clay nanocomposites. J Polym Sci, Part B: Polym Phys. 2004;42(22):4139–50.
Liu X, Wu Q. PP/clay nanocomposites prepared by grafting-melt intercalation. Polymer. 2001;42(25):10013–9.
Tang Y, Hu Y, Zhang R, Gui Z, Wang Z, Chen Z, et al. Investigation on polypropylene and polyamide-6 alloys/montmorillonite nanocomposites. Polymer. 2004;45(15):5317–26.
Mollova A, Androsch R, Mileva D, Gahleitner M, Funari SS. Crystallization of isotactic polypropylene containing beta-phase nucleating agent at rapid cooling. Eur Polymer J. 2013;49(5):1057–65. doi:10.1016/j.eurpolymj.2013.01.015.
Zheng W, Lu X, Ling Toh C, Hua Zheng T, He C. Effects of clay on polymorphism of polypropylene in polypropylene/clay nanocomposites. J Polym Sci, Part B: Polym Phys. 2004;42(10):1810–6.
Zhu S, Chen J, Zuo Y, Li H, Cao Y. Montmorillonite/polypropylene nanocomposites: mechanical properties, crystallization and rheological behaviors. Appl Clay Sci. 2011;52(1):171–8.
Chen K, Wilkie CA, Vyazovkin S. Nanoconfinement revealed in degradation and relaxation studies of two structurally different polystyrene-clay systems. J Phys Chem B. 2007;111(44):12685–92.
Papageorgiou DG, Tzounis L, Papageorgiou GZ, Bikiaris DN, Chrissafis K. β-nucleated propylene–ethylene random copolymer filled with multi-walled carbon nanotubes: mechanical, thermal and rheological properties. Polymer. 2014;55(16):3758–69.
Papageorgiou DG, Vourlias G, Bikiaris DN, Chrissafis K. Synergistic effect of functionalized silica nanoparticles and a β-nucleating agent for the improvement of the mechanical properties of a propylene/ethylene random copolymer. Macromol Mater Eng. 2014;299(6):707–21. doi:10.1002/mame.201300320.
Guth E. Theory of filler reinforcement. J Appl Phys. 2004;16(1):20–5.
Rooj S, Das A, Stöckelhuber KW, Wang D-Y, Galiatsatos V, Heinrich G. Understanding the reinforcing behavior of expanded clay particles in natural rubber compounds. Soft Matter. 2013;9(14):3798–808.
Wu Y-P, Jia Q-X, Yu D-S, Zhang L-Q. Modeling Young’s modulus of rubber–clay nanocomposites using composite theories. Polym Test. 2004;23(8):903–9.
Ishai O, Cohen LJ. Elastic properties of filled and porous epoxy composites. Int J Mech Sci. 1967;9(8):539–46.
Paul B. Trans Metall Soc AIME. 1960:36.
Fu S-Y, Feng X-Q, Lauke B, Mai Y-W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos B Eng. 2008;39(6):933–61.
Counto UJ. The effect of the elastic modulus of the aggregate on the elastic modulus, creep and creep recovery of concrete. Mag Concr Res. 1964;16(48):129–38.
Affdl J, Kardos J. The Halpin-Tsai equations: a review. Polym Eng Sci. 1976;16(5):344–52.
Chrissafis K, Bikiaris D. Can nanoparticles really enhance thermal stability of polymers? Part I: an overview on thermal decomposition of addition polymers. Thermochim Acta. 2011;523(1):1–24.
Bikiaris D. Can nanoparticles really enhance thermal stability of polymers? Part II: an overview on thermal decomposition of polycondensation polymers. Thermochim Acta. 2011;523(1–2):25–45.
Chen X-S, Yu Z-Z, Liu W, Zhang S. Synergistic effect of decabromodiphenyl ethane and montmorillonite on flame retardancy of polypropylene. Polym Degrad Stab. 2009;94(9):1520–5. doi:10.1016/j.polymdegradstab.2009.04.031.
Yu Z-Z, Yang M, Zhang Q, Zhao C, Mai Y-W. Dispersion and distribution of organically modified montmorillonite in nylon-66 matrix. J Polym Sci, Part B: Polym Phys. 2003;41(11):1234–43. doi:10.1002/polb.10480.
Papageorgiou D, Roumeli E, Chrissafis K, Lioutas C, Triantafyllidis K, Bikiaris D, et al. Thermal degradation kinetics and decomposition mechanism of PBSu nanocomposites with silica-nanotubes and strontium hydroxyapatite nanorods. Phys Chem Chem Phys. 2014;16(10):4830–42.
Sbirrazzuoli N. Determination of pre-exponential factors and of the mathematical functions f(α) or G(α) that describe the reaction mechanism in a model-free way. Thermochim Acta. 2013;564:59–69. doi:10.1016/j.tca.2013.04.015.
Šimon P, Thomas P, Dubaj T, Cibulková Z, Peller A, Veverka M. The mathematical incorrectness of the integral isoconversional methods in case of variable activation energy and the consequences. J Therm Anal Calorim. 2014;115(1):853–9. doi:10.1007/s10973-013-3459-7.
Friedman HL. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J Poly Sci Part C Polym Symp. 1964;6(1):183–95. doi:10.1002/polc.5070060121.
Acknowledgements
This research has been co-financed by the European Union (European Social Fund—ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF)—Research Funding Program: Heracleitus II. Investing in knowledge society through the European Social Fund. The authors appreciate financial support from the European Union under the Seventh Framework Program (Integrated Infrastructure Initiative No. 262348 European Soft Matter Infrastructure, ESMI).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Papageorgiou, D.G., Filippousi, M., Pavlidou, E. et al. Effect of clay modification on structure–property relationships and thermal degradation kinetics of β-polypropylene/clay composite materials. J Therm Anal Calorim 122, 393–406 (2015). https://doi.org/10.1007/s10973-015-4705-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4705-y