Abstract
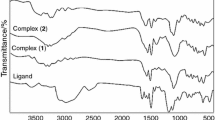
From the reaction of 1,2-di(o-aminophenylthio)ethane (dapte) and 5-bromosalicylaldehyde, Schiff base ligand N,N′-bis(5-bromosalicylaldehyde)-1,2-di(o-iminophenylthio)ethane ((Brsal)2dapte) was synthesized and characterized by elemental analyses (CHN), FT-IR, 13C and 1HNMR spectroscopy, as well as thermal analysis. Then, starting from (Brsal)2dapte, new complexes with copper(II) and nickel(II) were synthesized and characterized by elemental analyses (CHN) and FT-IR spectroscopy, as well as thermal analysis. FT-IR spectra of complexes indicate the deprotonated Schiff base ligand that coordinates with metal ions through azomethine nitrogen and phenolic oxygen. The thermal behavior of ligand and complexes provides useful information about the decomposition of all compounds in the subsequent steps. Nanoparticles of CuO and NiO were successfully prepared by solid-state thermal decomposition of the Schiff base complexes as novel precursor at 600 °C for 3 h without employing toxic solvent or surfactant and complicated equipment. The crystalline structures and morphology of final products were studied by X-ray powder diffraction and transmission electron microscopy.





Similar content being viewed by others
References
Taha ZA, Ajlouni A, Al-Mustafa J. Thermal decomposition of lanthanide(III) complexes of bis-(salicylaldehyde)-1,3-propylenediimine Schiff base ligand. Chem Pap. 2013;67:194–201.
Bal S, Bal SS, Erener A, Halipci HN, Akar S. Synthesis, thermal stability, electronic features, and antimicrobial activity of phenolic azo dyes and their Ni(II) and Cu(II) complexes. Chem Pap. 2014;68:352–61.
Paiva IL, de Carvalho GSC, da Silva AD, Corbi PP, Bergamini FRG, Formiga ALB, Diniz R, do Carmo WR, Leite CQF, Pavan FR, Cuin A. Silver(I)complexes with symmetrical Schiff bases: synthesis, structural characterization, DFT studies and antimycobacterial assays. Polyhedron. 2013;62:104–9.
Strianese M, Milione S, Bertolasi V, Pellecchia C. Iron and manganese pyridoxal-based complexes as fluorescent probes for nitrite and nitrate anions in aqueous solution. Inorg Chem. 2013;52:11778–86.
Khan MI, Khan A, Hussain I, Khan MA, Gul S, Iqbal M, Ur-Rahman I, Khuda F. Spectral, XRD, SEM and biological properties of new mononuclear Schiff base transition metal complexes. Inorg Chem Commun. 2013;35:104–9.
Alan I, Kriza A, Badea M, Stanica N, Olar R. Synthesis and characterization of Co(II), Ni(II), Zn(II) and Cd(II) complexes with 5-bromo-N, N’-bis-(salixylidene)-o-tolidine. J Therm Anal Calorim. 2013;111:483–90.
Oz S, Kurtaran R, Arici C, Ergun U, Kayay FND, Emregul KC, Atakol O, Ulku D. Two non-linear azide containing heteronuclear complexes: crystal structure and thermal decomposition. J Therm Anal Calorim. 2010;99:363–8.
Baran Y, Kaya I, Turkyilmaz M. Synthesis, spectroscopic and thermal properties of Pt(II) complexes of some polydentate ligands. J Therm Anal Calorim. 2012;107:869–75.
Khalaji AD, Nikookar M, Das D. Co(III), Ni(II), and Cu(II) complexes of bidentate N, O-donor Schiff base ligand derived from 4-methoxy-2-nitroaniline and salicylaldehyde. J Therm Anal Calorim. 2014;115:409–17.
Khalaji AD, Das D. Studies on Co(II) and Cu(II) complexes of a ligand derived from 1,3-phenylenediamine and 5-bromosalicylaldehyde: synthesis, characterization, thermal properties and use as new precursors for preparation cobalt and copper oxide nano-particles. J Therm Anal Calorim. 2013;114:671–5.
Chandra S, Kumar R. Synthesis and spectral studies on mononuclear complexes of chromium(III) and manganese(II) with 12-membered tetradentate N2O2, N2S2 and N4 donor macrocyclic ligands. Trans Met Chem. 2004;29:269–75.
Amirnasr M, Rasouli M, Mereiter K. Synthesis, crystal structures and electrochemical properties of CoIII complexes with hexadentate ligand containing thioether-amidopyridyl donor set. J Iran Chem Soc. 2013;10:275–82.
Montazerozohori M, Zahedi S, Naghiha A, Zohour MM. Synthesis, characterization and thermal behaviour of antibacterial and antifungal active zinc complexes of bis(3(4-dimethoxylaminophenyl)-allylidene-1,2-diaminoethane. Mater Sci Eng, C. 2014;35:195–204.
Montazerozohori M, Yadegari S, Naghiha A, Veyseh S. Synthesis, characterization, electrochemical behaviour, thermal study and antibacterial/antifungal properties of some new zinc(II) coordination compounds. J Ind Eng Chem. 2014;20:118–26.
Duan Y, Liu X, Han L, Asahina S, Xu D, Cao Y, Yao Y, Che S. Optically active chiral CuO nanoflowers. J Am Chem Soc. 2014;136:7193–6.
Jia X, Fan H, Yang W. Hydrothermal synthesis and primary gas sensing properties of CuO nanosheets. J Dis Technol. 2010;31:866–9.
Xu H, Huang J, Chen Y. Synthesis and characterization of porous CuO nanorods. Integr Ferroelectr. 2011;129:25–9.
Khalaji AD. Preparation and characterization of NiO nanoparticles via solid-state thermal decomposition of nickel(II) Schiff base complexes [Ni(salophen) and [Ni(Me-salophen)]. J Cluster Sci. 2013;24:209–15.
Saravanakumar S, Saravanan R, Sasikumar S. Effect of sintering temperature on the magnetic properties and charge density distribution of nano-NiO. Chem Pap. 2014;68:788–97.
Kim SI, Lee JS, Ahn HJ, Song HK, Jang JH. Dacile route to an efficient NiO supercapacitor with a three dimensional nanonetwork morphology. Appl Mater Interfaces. 2013;5:1596–603.
Acknowledgements
We are grateful to the Golestan University and the University of Burdwan for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khalaji, A.D., Das, D. Thermal stability of copper(II) and nickel(II) Schiff base complexes. J Therm Anal Calorim 120, 1529–1534 (2015). https://doi.org/10.1007/s10973-015-4534-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4534-z