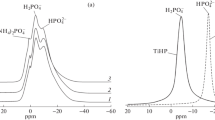
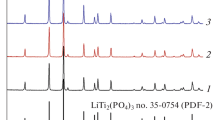
Abstract
The synthesis of titanium and lithium phosphates was studied using the sol-gel method. Particular attention was paid to phosphate precursors, which were mixtures of phosphate mono- and diesters prepared by solvolysis of P4O10 in isopropanol. The reaction of these precursors with titanium and lithium alkoxides yielded homogeneous gels and after drying and thermal cleavage of the esters at 300 °C, amorphous inorganic products. For the composition corresponding to the stoichiometric formula of a stable compound such as LiTi2(PO4)3, the phase crystallized as early as 550 °C by nucleation from the amorphous mixture. Ionic conductivity measured at room temperature was of the order of 10−5 S·cm−1 which increased after heat treatment at higher temperatures. If the composition did not correspond to a stable thermodynamic phase, phase separation occurred, and ionic conductivity decreased between 500 °C and 700 °C.
Graphical Abstract

Highlights
-
Lithium solid electrolyte LiTi2(PO4)3 was synthesized by sol-gel reaction.
-
Control of the reaction was performed by thermal cleavage of phosphate esters.
-
Same method was used to screen new lithium-titanium-phosphate compositions.
-
Lithium-ion conductivity was measured.













Similar content being viewed by others
References
Goodenough JB, Kim Y (2010) Challenges for rechargeable Li batteries. Chem Mater 22(3):587–603. https://doi.org/10.1021/cm901452z
Kim JG, Son B, Mukherjee S, Schuppert N, Bates A, Kwon O, Choi MJ, Chung HY, Park S (2015) A review of lithium and non-lithium based solid state batteries. J Power Sources 282:299–322. https://doi.org/10.1016/j.jpowsour.2015.02.054
Goodenough JB, Hong HY-P, Kafalas JA (1976) Fast Na+-ion transport in skeleton structures. Mater Res Bull 11(2):203–220. https://doi.org/10.1016/0025-5408(76)90077-5
Fu J (1997) Fast Li+ ion conduction in Li2O-Al2O3-TiO2-SiO2-P2O2 glass-ceramics. J Am Ceram Soc 80(7):1901–1903. https://doi.org/10.1111/j.1151-2916.1997.tb03070.x
Perthuis H, Colomban P (1986) Sol-gel routes leading to nasicon ceramics. Ceram Int 12(1):39–52. https://doi.org/10.1016/S0272-8842(86)80008-6
Bucharsky EC, Schell KG, Hintennach A, Hoffmann MJ (2015) Preparation and characterization of sol–gel derived high lithium ion conductive NZP-type ceramics Li1+x AlxTi2−x(PO4)3. Solid State Ion 274:77–82. https://doi.org/10.1016/j.ssi.2015.03.009
Deshpande A, Bansod S (2024) Effect of sintering temperature on sol-gel synthesized nasicon-type Li1.3Al0.3Ti1.7(PO4)3 ceramic solid electrolyte. J Mater Sci Mater Electron. https://doi.org/10.1007/s10854-023-11766-z
Livage J, Barboux P, Vandenborre MT, Schmutz C, Taulelle F (1992) Sol-gel synthesis of phosphates. J Non-Cryst Solids 147–148:18–23. https://doi.org/10.1016/S0022-3093(05)80586-1
Vasiliu I, Gartner M, Anastasescu M, Todan L, Predoana L, Elisa M, Negrila C, Ungureanu F, Logofatu C, Moldovan A, Birjega R, Zaharescu M (2007) Structural and Optical Properties of the SiO2–P2O5 Films Obtained by Sol–Gel Method. Thin Solid Films 515(16):6601–6605. https://doi.org/10.1016/j.tsf.2006.11.106
Lugmair CG, Tilley TD (1998) Di- Tert- butyl phosphate complexes of titanium. Inorg Chem 37(8):1821–1826. https://doi.org/10.1021/ic971347e
Paciorek KJL, Kratzer RH, Kaufman J, Nakahara JH, Christos T, Hartstein AM (1978) Thermal oxidative degradation studies of phosphate esters. Am Ind Hyg Assoc J 39(8):633–639. https://doi.org/10.1080/0002889778507827
Schmutz C, Basset E, Barboux P, Maquet J (1993) Study of titanium phosphate gels and their application to the synthesis of KTiOPO4 films. J Mater Chem 3(4):393. https://doi.org/10.1039/jm9930300393
Schmutz C, Basset E, Barboux P (1993) Couches minces de phosphates de titane par voie sol-gel. J Phys III 3(4):757–766. https://doi.org/10.1051/jp3:1993161
Takada K, Fujimoto K, Inada T, Kajiyama A, Kouguchi M, Kondo S, Watanabe M (2002) Sol- Gel Preparation of Lithium Ion Conductive Thin Film. Appl Surf Sci 89:300–306. https://doi.org/10.1016/S0169-4332(01)01007-8
Kunshina GB, Gromov OG, Lokshin EP, Kalinnikov VT (2014) Sol-gel synthesis of Li1.3Al0.3Ti1.7(PO4)3 solid electrolyte. Russ J Inorg Chem 59(5):424–430. https://doi.org/10.1134/S0036023614050118
Qu X, Yu Z, Ruan D, Dou A, Su M, Zhou Y, Liu Y, Chu D (2020) Enhanced electrochemical performance of Ni-rich cathode materials with Li 1.3 Al 0.3 Ti 1.7 (PO 4) 3 coating. ACS Sustain Chem Eng 8(15):5819–5830. https://doi.org/10.1021/acssuschemeng.9b05539
Rodriguez-carvajal J (1993) Recent advances in magnetic structure determination neutron powder diffraction. Phys B 192:55–69
La Monaca A, Girard G, Savoie S, Demers H, Bertoni G, Krachkovskiy S, Marras S, Mugnaioli E, Gemmi M, Benetti D, Vijh A, Rosei F, Paolella A (2021) Effect of pressure on the properties of a NASICON Li1.3Al0.3Ti1.7(PO4)3 nanofiber solid electrolyte. J Mater Chem A 9(23):13688–13696. https://doi.org/10.1039/D1TA01143J
Kuhlman R, Vaartstra BA, Streib WE, Huffman JC, Caulton KG (1993) Primary steps in the hydrolyses of two heterometallic alkoxides. Characterization of lithium-titanium and barium-zirconium isopropoxides [LiTiO(O-Iso-Pr)3]4 and BaZr4(OH)(O-Iso-Pr)17. Inorg Chem 32(7):1272–1278. https://doi.org/10.1021/ic00059a040
Cretin M, Fabry P, Abello L (1996) ChemInform Abstract: Li1+xAlxTi2-x(PO4)3 for Li+ potentiometric sensors. ChemInform. https://doi.org/10.1002/chin.199606010.
Arbi K, Kuhn A, Sanz J, García-Alvarado F (2010) Characterization of lithium insertion into NASICON-type Li1 + x Ti2 − x Al x (PO4) 3 and its electrochemical behavior. J Electrochem Soc 157(6):A654. https://doi.org/10.1149/1.3368764
Lange FF, Balmer ML, Levi CG (1994) Diffusion limited crystallization and phase partitioning in ZrO2-metal oxide binary systems: code: D3. J Sol Gel Sci Technol 2(1–3):317–321. https://doi.org/10.1007/BF00486263
Barboux P, Griesmar P, Ribot F, Rolles L (1995) Homogeneity-related problems in solution derived powders. J Solid State Chem 117(2):343–350. https://doi.org/10.1006/jssc.1995.1283
Ponnala B, Balla P, Hussain SK, Ginjupalli SR, Koppadi K, Nekkala N, Perupogu V, Lassi U, Seelam PK (2022) Selective hydrogenolysis of biodiesel waste bioglycerol over titanium phosphate (TiP) catalysts: the effect of Pt & WO3 loadings. Waste Biomass- Valoriz 13(11):4389–4402. https://doi.org/10.1007/s12649-022-01909-4
Rao KJ, Sobha KC, Kumar S (2001) Infrared and Raman spectroscopic studies of glasses with NASICON-type chemistry. J Chem Sci 113(5–6):497–514. https://doi.org/10.1007/BF02708786
Ayu NIP, Kartini E, Prayogi LD, Faisal M, Supardi (2016) Crystal structure analysis of Li3PO4 powder prepared by wet chemical reaction and solid-state reaction by using X-ray diffraction (XRD). Ionics 22(7):1051–1057. https://doi.org/10.1007/s11581-016-1643-z
Kanno R, Murayama M (2001) Lithium ionic conductor Thio-LISICON: the Li[Sub 2]S-GeS[Sub 2]-P[Sub 2]S[Sub 5] system. J Electrochem Soc 148(7):A742. https://doi.org/10.1149/1.1379028
Acknowledgements
NMR equipment at ESPCI Paris PSL is funded in part by the Paris Region. We thank Agence Nationale de la Recherche et de la Technologie for the financial support of A. Guillot during thesis contract CIFRE n°2020/1330.
Author information
Authors and Affiliations
Contributions
AG performed the synthesis characterization during his PhD work, helped by AS during her master’s internship. MNR performed the liquid-state NMR characterizations at Chimie Paristech and J-BD the solid-state NMR experiments at ESPCI. CM and XR supervised the work on the industrial site while DG and PB supervised the PhD and academic work. AG and PB prepared the figures. PB, AG, and DG wrote the main manuscript text. All authors reviewed the document.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guillot, A., Giaume, D., Suvorova, A. et al. Synthesis of lithium conducting titanium phosphates by the sol-gel process. J Sol-Gel Sci Technol (2024). https://doi.org/10.1007/s10971-024-06461-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10971-024-06461-2