Abstract
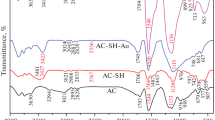
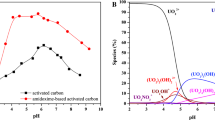
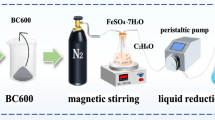
In this paper, cheap liquorice residue was used to prepare activated carbon (AC), thioacetamide (TAA) was used to modify the AC, and the adsorption experiments were conducted in the simulated acid radioactive wastewater with low uranium concentration to study the adsorption behavior and mechanism for uranium by TAA modified AC (TAA–AC). The removal efficiency by TAA–AC was 92.1–98.2% from the 1 mg L−1 uranium solution at pH 2–6. The adsorption equilibrium data were well fitted by Dubinin–Radushkevich model, and the maximum adsorption capacity was estimated to be 340 mg g−1. TAA–AC showed an enhanced selectivity for uranium in the presence of competitive ions. Furthermore, the adsorption experiments were conducted in the actual acid radioactive wastewater with low uranium concentration from an in situ leach uranium mine. The high adsorption rate (98.3%) and selectivity (Kd = 3.78×104 mL g−1) for uranium were observed in the actual acid radioactive wastewater, and the adsorption rate was found to maintain 96.2% over six cycles of adsorption–desorption.










Similar content being viewed by others
References
Wang X, Wang T, Zheng X, Shen Y, Lu X (2017) Isotherms, thermodynamic and mechanism studies of removal of low concentration uranium(VI) by Aspergillus niger. Water Sci Technol 75(12):2727–2736
Abdi S, Nasiri M, Mesbahi A, Khani MH (2017) Investigation of uranium(VI) adsorption by polypyrrole. J Hazard Mater 332:132–139
Bayramoglu G, Celik G, Arica MY (2006) Studies on accumulation of uranium by fungus Lentinus sajor-caju. J Hazard Mater 136(2):345–353
Wang G, Liu J, Wang X, **e Z, Deng N (2009) Adsorption of uranium(VI) from aqueous solution onto cross-linked chitosan. J Hazard Mater 168(2–3):1053–1058
Favas PJ, Pratas J, Mitra S, Sarkar SK, Venkatachalam P (2016) Biogeochemistry of uranium in the soil-plant and water-plant systems in an old uranium mine. Sci Total Environ 568:350–368
Chen C, Wang J (2016) Uranium removal by novel graphene oxide-immobilized Saccharomyces cerevisiae gel beads. J Environ Radioact 162–163:134–145
Kong L, Su M, Mai Z, Li H, Diao Z, **ong Y, Chen D (2017) Removal of uranium from aqueous solution by two-dimensional electrosorption reactor. Environ Technol Innov 8:57–63
Li C, Wei Y, Wang X, Yin X (2018) Efficient and rapid adsorption of iodide ion from aqueous solution by porous silica spheres loaded with calcined Mg–Al layered double hydroxide. J Taiwan Inst Chem Eng 85:193–200
Paschalidou P, Liatsou I, Pashalidis I, Theocharis CR (2017) Effect of surface and textural characteristics on uranium adsorption by nanoporous titania. J Radioanal Nucl Chem 314(2):1141–1147
Ghasemi Torkabad M, Keshtkar AR, Safdari SJ (2017) Comparison of polyethersulfone and polyamide nanofiltration membranes for uranium removal from aqueous solution. Prog Nucl Energy 94:93–100
Chen L, Chen Y, Wang X, Wei Y, He L, Tang F (2017) A novel silica-based anion exchange resin used for removing uranium from drinking water. J Radioanal Nucl Chem 314(3):2569–2578
Yu J, Wang J, Jiang Y (2017) Removal of uranium from aqueous solution by alginate beads. Nucl Eng Technol 49(3):534–540
Nekhunguni PM, Tavengwa NT, Tutu H (2017) Sorption of uranium(VI) onto hydrous ferric oxide-modified zeolite: assessment of the effect of pH, contact time, temperature, selected cations and anions on sorbent interactions. J Environ Manag 204(Pt 1):571–582
Dangelmayr MA, Reimus PW, Wasserman NL, Punsal JJ, Johnson RH, Clay JT, Stone JJ (2017) Laboratory column experiments and transport modeling to evaluate retardation of uranium in an aquifer downgradient of a uranium in situ recovery site. Appl Geochem 80:1–13
Wang F, Liu Q, Li R, Li Z, Zhang H, Liu L, Wang J (2016) Selective adsorption of uranium(VI) onto prismatic sulfides from aqueous solution. Colloids Surf A 490:215–221
Gao X, Bi M, Shi K, Chai Z, Wu W (2017) Sorption characteristic of uranium(VI) ion onto K-feldspar. Appl Radiat Isot 128:311–317
Anirudhan TS, Nima J, Divya PL (2015) Adsorption and separation behavior of uranium(VI) by 4-vinylpyridine-grafted-vinyltriethoxysilane-cellulose ion imprinted polymer. J Environ Chem Eng 3(2):1267–1276
Bhargava SK, Ram R, Pownceby M, Grocott S, Ring B, Tardio J, Jones L (2015) A review of acid leaching of uraninite. Hydrometallurgy 151:10–24
Miśkiewicz A, Zakrzewska-Kołtuniewicz G, Dłuska E, Walo PF (2016) Application of membrane contactor with helical flow for processing uranium ores. Hydrometallurgy 163:108–114
Douglas G, Shackleton M, Woods P (2014) Hydrotalcite formation facilitates effective contaminant and radionuclide removal from acidic uranium mine barren lixiviant. Appl Geochem 42:27–37
Perdrial N, Vázquez-Ortega A, Wang G, Kanematsu M, Mueller KT, Um W, Steefel CI, O’Day PA, Chorover J (2018) Uranium speciation in acid waste-weathered sediments: the role of aging and phosphate amendments. Appl Geochem 89:109–120
Solgy M, Taghizadeh M, Ghoddocynejad D (2015) Adsorption of uranium(VI) from sulphate solutions using Amberlite IRA-402 resin: equilibrium, kinetics and thermodynamics study. Ann Nucl Energy 75:132–138
Ölmez Ş, Eral M (1994) Extraction of uranium from acidic solutions by TBP impregnated polyurethane foam. In: Kučera J, Obrusník I, Sabbioni E (eds) Nuclear analytical methods in the life sciences. Humana Press, Totowa, pp 731–735
Hussein AEM, Taha MH (2012) Uranium removal from nitric acid raffinate solution by solvent immobilized PVC cement. J Radioanal Nucl Chem 295(1):709–715
Morsy AMA, Hussein AEM (2011) Adsorption of uranium from crude phosphoric acid using activated carbon. J Radioanal Nucl Chem 288(2):341–346
Oyewo OA, Onyango MS, Wolkersdorfer C (2016) Application of banana peels nanosorbent for the removal of radioactive minerals from real mine water. J Environ Radioact 164:369–376
Zhang G, Qu J, Liu H, Cooper AT, Wu R (2007) CuFe2O4/activated carbon composite: a novel magnetic adsorbent for the removal of acid orange II and catalytic regeneration. Chemosphere 68(6):1058–1066
Abbasi A, Streat M (1998) Sorption of uranium from nitric acid solution using Tbp-impregnated activated carbons. Solvent Extr Ion Exchange 16(5):1303–1320
Enniya I, Rghioui L, Jourani A (2018) Adsorption of hexavalent chromium in aqueous solution on activated carbon prepared from apple peels. Sustain Chem Pharm 7:9–16
Hong S, Cannon FS, Hou P, Byrne T, Nieto-Delgado C (2017) Adsorptive removal of sulfate from acid mine drainage by polypyrrole modified activated carbons: effects of polypyrrole deposition protocols and activated carbon source. Chemosphere 184:429–437
Chen S, Hong J, Yang H, Yang J (2013) Adsorption of uranium (VI) from aqueous solution using a novel graphene oxide-activated carbon felt composite. J Environ Radioact 126:253–258
Al-Malack MH, Dauda M (2017) Competitive adsorption of cadmium and phenol on activated carbon produced from municipal sludge. J Environ Chem Eng 5(3):2718–2729
Kaghazchi T, Kolur NA, Soleimani M (2010) Licorice residue and Pistachio-nut shell mixture: a promising precursor for activated carbon. J Ind Eng Chem 16(3):368–374
Kaghazchi T, Soleimani M, Yeganeh MM (2006) Production of activated carbon from residue of liquorices chemical activation. In: 8th Asia-Pacific international symposium on combustion and energy utilization, Sochi, Russian. ISBN 5-89238-086-6
Ding DX, **n X, Li L, Hu N, Li GY, Wang YD, Fu PK (2014) Removal and recovery of U(VI) from low concentration radioactive wastewater by ethylenediamine-modified biomass of Aspergillus niger. Water Air Soil Pollut 225(12):2206
Li L, Hu N, Ding D, **n X, Wang Y, Xue J, Zhang H, Tan Y (2015) Adsorption and recovery of U(VI) from low concentration uranium solution by amidoxime modified Aspergillus niger. RSC Adv 5(81):65827–65839
Jaouadi M, Hbaieb S, Guedidi H, Reinert L, Amdouni N, Duclaux L (2017) Preparation and characterization of carbons from β-cyclodextrin dehydration and from olive pomace activation and their application for boron adsorption. J Saudi Chem Soc 21(7):822–829
Husnain SM, Kim HJ, Um W, Chang YY, Chang YS (2017) Superparamagnetic adsorbent based on phosphonate grafted mesoporous carbon for uranium removal. Ind Eng Chem Res 56(35):9821–9830
Si ZX, Xu W, Zheng YQ (2016) Synthesis, structure, luminescence and photocatalytic properties of an uranyl-2, 5-pyridinedicarboxylate coordination polymer. J Solid State Chem 239:139–144
Sessler JL, Seidel D, Vivian AE, Lynch V, Scott BL, Keogh DW (2001) Hexaphyrin (1.0. 1.0. 0.0): an expanded porphyrin ligand for the actinide cations uranyl (UO2 2+) and neptunyl (NpO2 +). Angew Chem Int Ed 40(3):591–594
Unruh DK, Libo A, Streicher L, Forbes TZ (2014) Synthesis and characterization of 1-d uranyl thiodigycolate coordination polymers. Polyhedron 73:110–117
Mezaguer M, Neh K, Lounici H, Kamel Z (2012) Characterization and properties of Pleurotus mutilus fungal biomass as adsorbent of the removal of uranium(VI) from uranium leachate. J Radioanal Nucl Chem 295(1):393–403
Naeem H, Bhatti HN, Sadaf S, Iqbal M (2017) Uranium remediation using modified Vigna radiata waste biomass. Appl Radiat Isot 123:94–101
Cheira MF, Atia BM, Kouraim MN (2017) Uranium(VI) recovery from acidic leach liquor by Ambersep 920U SO 4 resin: kinetic, equilibrium and thermodynamic studies. J Radiat Res Appl Sci 10(4):307–319
Ahmed SH, Sharaby CM, El Gammal EM (2013) Uranium extraction from sulfuric acid medium using trioctylamine impregnated activated carbon. Hydrometallurgy 134–135:150–157
Li B, Ma L, Tian Y, Yang X, Li J, Bai C, Yang X, Zhang S, Li S, ** Y (2014) A catechol-like phenolic ligand-functionalized hydrothermal carbon: one-pot synthesis, characterization and sorption behavior toward uranium. J Hazard Mater 271:41–49
Zhao C, Liu J, Tu H, Li F, Li X, Yang J, Liao J, Yang Y, Liu N, Sun Q (2016) Characteristics of uranium biosorption from aqueous solutions on fungus Pleurotus ostreatus. Environ Sci Pollut Res Int 23(24):24846–24856
Saleh TA, Tuzen M, Sarı A (2017) Polyethylenimine modified activated carbon as novel magnetic adsorbent for the removal of uranium from aqueous solution. Chem Eng Res Des 117:218–227
Mahmoud ME, Khalifa MA, El Wakeel YM, Header MS, Abdel-Fattah TM (2017) Engineered nano-magnetic iron oxide-urea-activated carbon nanolayer sorbent for potential removal of uranium(VI) from aqueous solution. J Nucl Mater 487:13–22
Zhao Y, Liu C, Feng M, Chen Z, Li S, Tian G, Li S (2010) Solid phase extraction of uranium(VI) onto benzoylthiourea-anchored activated carbon. J Hazard Mater 176(1–3):119–124
Liu Y, Dai Y, Yuan D, Wang Y, Zou L (2017) The preparation of PZS-OH/CNT composite and its adsorption of U(VI) in aqueous solutions. J Radioanal Nucl Chem 314(3):1747–1757
Bulut Y, Tez Z (2007) Removal of heavy metals from aqueous solution by sawdust adsorption. J Environ Sci 19(2):160–166
Acknowledgements
This research was supported by the National Natural Science Foundation of China (U1401231, 11405081 and 51704170), the Development Program for Science and Technology for National Defense (B3720132001), the China Postdoctoral Science Foundation (2017M612569), and the Research Foundation of Education Bureau of Hunan Province (16C1386).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tan, Y., Li, L., Zhang, H. et al. Adsorption and recovery of U(VI) from actual acid radioactive wastewater with low uranium concentration using thioacetamide modified activated carbon from liquorice residue. J Radioanal Nucl Chem 317, 811–824 (2018). https://doi.org/10.1007/s10967-018-5952-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-5952-8