Abstract
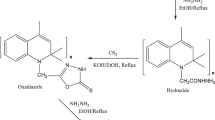
The commonly used phenolic resin vulcanizing agents for rubber processing technology have some limitations. In this study, a new aldehyde teroctyl phenolic resin vulcanizing agent was synthesized from SP-1055 through a series of chemical reactions and tested for its efficiency in vulcanizing isoprene rubber (IR) at low dosages. The structure of the new phenolic resin was confirmed using infrared spectroscopy (FTIR) and 1H nuclear magnetic resonance (1H-NMR) spectroscopy. The results showed that the new phenolic resin exhibited excellent vulcanization properties and environmental protection characteristics, and the IR blended with the new phenolic resin vulcanizing agent showed higher tensile strength, elongation at break, and better recovery from prolonged extension than those of IR with traditional phenolic resin vulcanizing agents. The improvement is attributed to the replacement of the chain end hydroxyl with an aldehyde group in the new phenolic resin vulcanizing agent. The new phenolic resin vulcanizing agent has significant potential for future research and development and could be a promising alternative for medical rubber products.




















Similar content being viewed by others
Data Availability Statement
The datasets used or analysed during the current study are available from the corresponding author on reasonable request.
Reference
Ghosh P, Katare S, Patkar P, Caruthers JM, Venkatasubramanian V, Walker KA (2003) Sulfur Vulcanization of Natural Rubber for Benzothiazole Accelerated Formulations: From Reaction Mechanisms to a Rational Kinetic Model. Rubber Chem Technol 76:592–693
Qi T, Chengzhong S (2020) Study on properties of Dynamic vulcanized butyl rubber/Polypropylene thermoplastic vulcanized Rubber with octyl phenolic resin. Rubber Sci Technol 18:376–380
Yang Q (2015) Modern Rubber Technology. Bei**g: China Petrochemical Press 136–137
Wang DX, Lu C, Wang B et al (2020) Kinetics of phenolic resin curing nitrile butadiene rubbe. Synthetic Rubber Ind 43(3):230–235
Sun LY et al (2021) Hydrometallurgical recycling of valuable metals from spent lithium-ion batteries by reductive leaching with stannous chloride. Int J Minerals Metallurgy Mater 28(6):991–1000
Zhuang W et al (2020) Comparative Acute Toxicity and Oxidative Stress Responses in Three Aquatic Species Exposed to Stannic Oxide Nanoparticles and Stannic Chloride. Bull Environ Contam Toxicol 105(6):841–846
**angren Z (1989) Preparation of brominated p-teroctyl phenolic resin. Shanxi Chem Ind 1:15–19
Liu Dachen D, Minghui WH (2022) Bromination modification of phenolic hydroxyl sites of crosslinked teroctyl phenolic resin. Chem prog 01:1–8
Robert P, Lattimer RA, Kinsey RW, Layer CKR (1989) The Mechanism of Phenolic Resin Vulcanization of Unsaturated Elastomers. Rubber Chem Technol 62(1):107–123
Nakason C, Nuansomsri K, Kaesaman A, Kiatkamjornwong S (2006) Dynamic vulcanization of natural rubber/high-density polyethylene blends: Effect of compatibilization, blend ratio and curing system. Polym Test 25:782–796
Maria V, Papadopoulou EC (2021) Taylor, Intramolecular Diels-Alder reactions of 1, 2, 4-triazines. Synthesis of 3-alkylpyridines via Raney nickel desulfurization of thieno [2,3-b]pyridines. Tetrahedron 4(89):132158
Williams DR, Gaston RD, Horton III IB (1985) Intramolecular Diels-alder cycloadditions of bis-diene substrates. Tetrahedron Lett 26(11):1391–1394
Houwink RJT (1958) Kinetics of the formation of conjugated dienes. J Polym Sci 30(126):449–464
LiuYuyana FY, ZhangLia JX, Ruifeng L, ZhuShuairua GH, FangJianghuaa X Q (2014) Advances in LiCl-Promoted Preparation of Polyfunctional Grignard Reagents and the Applications. Chinese J Org Chem 34(08):1523–1541
Longhu Z, Toshikazu H (2000) Vanadium-catalyzed stereoselective cyclodimerization of arylidene malononitrile in the presence of chlorosilane and zinc. Tetrahedron Lett 41(44):8517–8521
Blum J, Christ W (1963) Protection of Hydroxyl Groups as tert-Butyldimethylsilyl Derivatives. J Org Chem 28(12):3555–3559
Yoshiaki H, Satoshi M, Eiichi N, Isao K (1986) Me3SiCl/HMPA accelerated conjugate addition of catalytic copper reagent. Stereoselective synthesis of enol silyl ether of aldehyde. Tetrahedron Lett 27(34):4025–4028
Nakamura E, Matsuzawa S, Horiguchi Y, Kuwajima I (1986) Me3SiCl accelerated conjugate addition of stoichiometric organocopper reagents. Tetrahedron Lett 27(34):4029–4032
Paul K (2005) In Handbook of Functionalized Organometallics. Wiley-VCH 109
Yingbin L (2019) Preparation and application analysis of Grignard reagent. Contemporary Chem Res 7:18–19
Petrzilka TH, Demuth M, Lusuardi WG (1973) Synthesis of hashish constituents. Helv Chim Acta 56(1):519–24
Weimei Q, Wenhong Z (2010) Preparation and application of Grignard reagent. Henan Chem Ind 27(14):20–21
Cuiju Z, Rositha K, Lutz A (2019) Manganese(I)-Catalyzed C-H Activation/Diels-Alder/retro-Diels-Alder Domino Alkyne Annulation featuring Transformable Pyridines. Angew Chem 58(16):5338–5342
Funding
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Du, M., Liu, D. Synthesis and characterization of a novel aldehyde teroctyl phenolic resin vulcanizing agent for isoprene rubber processing. J Polym Res 30, 249 (2023). https://doi.org/10.1007/s10965-023-03622-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-023-03622-9