Abstract
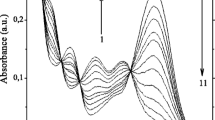
The supramolecular complex formation between donor (E)-bis(18-crown-6)azobenzene ((E)-1) and bis(ammoniopropyl) derivatives of bipyridine and dipyridylethylene acceptors (2, (E)-3) was studied in MeCN using steady-state absorption and fluorescence spectroscopy, fluorescence kinetics, and quantum-chemical (DFT, TDDFT, QDPT) calculations. The complexes are quite stable, but they undergo decomposition via substitution by alkali and alkaline-earth metal cations. The formation of 1:1 and 1:2 complexes between (E)-1 and alkali and alkaline-earth metal cations (Mn+) was confirmed. Spectral-kinetic parameters and stability constants were calculated. The determined stability constants are generally lower than those for bis(crown)stilbene complexes due to moderate electron-donor ability of azobenzene. The plot of fluorescence quantum yields of 1·(Mn+)2 vs charge density of the metal cation shows two separate linear relations for unipositive and dipositive ions. The results of quantum-chemical calculations supported the conclusions derived from steady-state experiments. The most stable conformations of pseudocyclic complex (E)-1·2 and two metal complexes were found. The latter involve 4 or 6 molecules of acetonitrile per each complex: (E)-1·(Ca2+)2·(MeCN)4, (E)-1·(K+)2·(MeCN)6. Based upon quantum-chemical calculations of model species, the character of absorption bands of crowns and their complexes with actual energy conversion pathway are discussed.







Similar content being viewed by others
References
Gromov, S.P., Vedernikov, A.I., Ushakov, E.N., Lobova, N.A., Botsmanova, A.A., Basok, S.S., Kuzʹmina, L.G., Churakov, A.V., Strelenko, Y.A., Alfimov, M.V., Ivanov, E.I., Howard, J.A.K., Johnels, D., Edlund, U.G.: Novel supramolecular charge-transfer systems based on bis(18-crown-6)stilbene and viologen analogues bearing two ammonioalkyl groups. New J. Chem. 29, 881–894 (2005). https://doi.org/10.1039/B500667H
Vedernikov, A.I., Kuzʹmina, L.G., Botsmanova, A.A., Strelenko, Yu.A., Howard, J.A.K., Alfimov, M.V., Gromov, S.P.: Stacking structures of complexes between bis(crown)azobenzene and a dipyridylethylene derivative in a crystal and in solution. Mendeleev Comm. 17, 148–150 (2007). https://doi.org/10.1016/j.mencom.2007.05.005
Ushakov, E.N., Martyanov, T.P., Vedernikov, A.I., Efremova, A.A., Moiseeva, A.A., Kuzʹmina, L.G., Dmitrieva, S.N., Howard, J.A.K., Gromov, S.P.: Highly stable supramolecular donor–acceptor complexes involving a bis(18-crown-6)azobenzene as weak donor: structure–property relationships. ACS Omega 5, 25993–26004 (2020). https://doi.org/10.1021/acsomega.0c03441
Shinkai, S., Ogawa, T., Kusano, Y., Manabe, O.: Selective extraction of alkali metal cations by a photoresponsive bis(crown ether). Chem. Lett. 9(3), 283–286 (1980). https://doi.org/10.1246/cl.1980.283
Ushakov, E.N., Alfimov, M.V., Gromov, S.P.: Crown ether-based optical molecular sensors and photocontrolled ionophores. Macroheterocycles 3(4), 189–200 (2010). https://doi.org/10.6060/mhc2010.4.189
Cacciapaglia, R., Stefano, S., Di, M.L.: The bis-barium complex of a butterfly crown ether as a phototunable supramolecular catalyst. J. Am. Chem. Soc. 125(8), 2224–2227 (2003). https://doi.org/10.1021/ja029331x
Dorel, R., Feringa, B.L.: Photoswitchable catalysis based on the isomerisation of double bonds. Chem. Comm. 55, 6477–6486 (2019). https://doi.org/10.1039/C9CC01891C
Pang, J., Ye, Y., Tian, Z., Pang, X., Wu, Ch.: Theoretical insight into azobis-(benzo-18-crown-6) ether combined with the alkaline earth metal cations. Comp. Theoret. Chem. 1066, 28–33 (2015). https://doi.org/10.1016/j.comptc.2015.04.012
Demas, J.N., Crosby, G.A.: The measurement of photoluminescence quantum yields: a review. J. Phys. Chem. 75(8), 991–1024 (1971). https://doi.org/10.1021/j100678a001
Himmelblau, D.M.: Applied nonlinear programming. Mc-Grow-Hill, Austin (1972)
Vedernikov, A.I., Basok, S.S., Gromov, S.P., Kuzmina, L.G., Avakyan, V.G., Lobova, N.A., Kulygina, E.Y., Titkov, T.V., Strelenko, Y.A., Ivanov, E.I., Howard, J.A.K., Alfimov, M.V.: Synthesis and structure of bis-crown-containing stilbenes. Russ. J. Org. Chem. 41, 843–854 (2005). https://doi.org/10.1007/s11178-005-0255-2
Vedernikov, A.I., Ushakov, E.N., Efremova, A.A., Kuzʹmina, L.G., Moiseeva, A.A., Lobova, N.A., Churakov, A.V., Strelenko, Y.A., Alfimov, M.V., Howard, J.A.K., Gromov, S.P.: Synthesis, structure, and properties of supramolecular charge-transfer complexes between bis(18-crown-6)stilbene and ammonioalkyl derivatives of 4,4ʹ-bipyridine and 2,7-diazapyrene. J. Org. Chem. 76(16), 6768–6779 (2011). https://doi.org/10.1021/jo201172w
Noureldin, N.A., Bellegarde, J.W.: A novel method. The synthesis of ketones and azobenzenes using supported permanganate. Synthesis 6, 939–942 (1999)
Schmidt, M.W., Baldridge, K.K., Boatz, J.A., Elbert, S.T., Gordon, M.S., Jensen, J.H., et al.: General atomic and molecular electronic structure system. J. Comput. Chem. 14, 1347–1363 (1993). https://doi.org/10.1002/jcc.540141112
Marenich, A.V., Cramer, C.J., Truhlar, D.G.: Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113(18), 6378–6396 (2009). https://doi.org/10.1021/jp810292n
Granovsky A. A.: Firefly 8.2, build 10203 (2017). https://classic.chem.msu.su/gran/Firefly/index.html
Granovsky, A.A.: Extended multi-configuration quasidegenerate perturbation theory: the new approach to multi-state multi-reference perturbation theory. Chem. Phys. 134, 214113–214126 (2011). https://doi.org/10.1063/1.3596699
Miertus, S., Scrocco, E., Tomasi, J.: Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chem. Phys. 55, 117–129 (1981). https://doi.org/10.1016/0301-0104(81)85090-2
Shinkai, S., Ogawa, T., Kusano, Y., Manabe, O., Kikukawa, K., Goto, T., Matsuda, T.: Photoresponsive crown ethers. Influence of alkali metal cations on photoisomerization and thermal isomerization of azobis(benzocrown ethers). J. Am. Chem. Soc. 104, 1960–1967 (1982). https://doi.org/10.1021/ja00371a027
Abakumova, N.I., Kolenko, I.P., Kodess, M.I.: Synthesis of nitro- and amino derivatives of a macrocyclic polyester of 2,3-benzo-1,4,7,10,13,16-hexaoxocyclooctadeca-2-ene. Zhurnal Organicheskoy Khimii (in Russian) 18, 1495 (1982). https://doi.org/10.1002/chin.198246242
Rusalov, M.V., Volchkov, V.V., Ivanov, V.L., Melnikov, M.Y., Shelaev, I.V., Gostev, F.E., Nadtochenko, V.A., Vedernikov, A.I., Gromov, S.P., Alfimov, M.V.: Femtosecond excited state dynamics of a stilbene–viologen charge transfer complex assembled via host–guest interaction. Photochem. Photobiol. Sci. 16(12), 1801–1811 (2017). https://doi.org/10.1039/c7pp00170c
Volchkov, V.V., Khimich, M.N., Rusalov, M.V., Gostev, F.E., Shelaev, I.V., Nadtochenko, V.A., Vedernikov, A.I., Gromov, S.P., Freidzon, A.Y., Alfimov, M.V., Melnikov, M.Y.: Formation of a supramolecular charge-transfer complex. Ultrafast excited state dynamics and quantum-chemical calculations. Photochem. Photobiol. Sci. 18, 232–241 (2019). https://doi.org/10.1039/c8pp00328a
Ushakov, E.N., Nadtochenko, V.A., Gromov, S.P., Vedernikov, A.I., Lobova, N.A., Alfimov, M.V., Gostev, F.E., Petrukhin, A.N., Sarkisov, O.M.: Ultrafast excited state dynamics of the bi- and termolecular stilbene-viologen charge-transfer complexes assembled via host-guest interactions. Chem. Phys. 298, 251–261 (2004). https://doi.org/10.1016/j.chemphys.2003.12.002
Volchkov, V.V., Gostev, F.E., Shelaev, I.V., Nadtochenko, V.A., Dmitrieva, S.N., Gromov, S.P., Melnikov, M.Y.: Complexation of donor-acceptor substituted aza-crowns with alkali and alkaline earth metal cations. Charge transfer and recoordination in excited state. J. Fluoresc. 26(2), 585–592 (2016). https://doi.org/10.1007/s10895-015-1744-5
Valeur, B., Bourson, J., Pouget, J.: In A. W. Czarnik (ed) Fluorescent chemosensors for ion and molecular recognition. ACS Symposium Series 538, Washington (1993)
Volchkov, V.V., Rusalov, M.V., Gostev, F.E., Shelaev, I.V., Nadtochenko, V.A., Vedernikov, A.I., Efremova, A.A., Kuzmina, L.G., Gromov, S.P., Alfimov, M.V., Melnikov, M.Y.: Complexation of bis-crown stilbene with alkali and alkaline-earth metal cations. Ultrafast excited state dynamics of the stilbene-viologen analogue charge transfer complex. J. Phys. Org. Chem. 31(2), e3759 (2018). https://doi.org/10.1002/poc.3759
Wallace, R.M., Katz, S.M.: Method for the determination of rank in the analysis of absorption spectra of multicomponent systems. J. Phys. Chem. 68(12), 3890–3892 (1964). https://doi.org/10.1021/j100794a511
Rurack, K., Bricks, J.L., Reck, G., Radeglia, R., Resch-Genger, U.: Chalcone-analogue dyes emitting in the near-infrared (NIR): influence of donor–acceptor substitution and cation complexation on their spectroscopic properties and X-ray structure. J. Phys. Chem. A 104(14), 3087–3109 (2000). https://doi.org/10.1021/jp994269k
Freidzon, A.Y., Vladimirova, K.G., Bagatur’Yants, A.A., Gromov, S.P., Alfimov, M.V.: Theoretical study of complexation of alkali metal ions in the cavity of arylazacrown ethers. J. Molec. Struct.: THEOCHEM. 809, 61–71 (2007). https://doi.org/10.1016/j.theochem.2007.01.033
Freidzon, A.Y., Bagatur’Yants, A.A., Gromov, S.P., Alfimov, M.V.: Recoordination of a metal ion in the cavity of a crown compound: a theoretical study. Effect of the metal ion-solvent interaction on the conformations of calcium complexes of arylazacrown ethers. Russ. Chem. Bull. 9, 2042–2054 (2005). https://doi.org/10.1007/s11172-006-0076-7
Freidzon, A.Y., Bagatur’Yants, A.A., Gromov, S.P., Alfimov, M.V.: Recoordination of a metal ion in the cavity of an arylazacrown ether: model study of the conformations and microsolvation of calcium complexes of arylazacrown ethers. Int. J. Quant. Chem. 100, 617–625 (2004). https://doi.org/10.1002/qua.20184
Atabekyan, L.S., Freidzon, A.Y., Chibisov, A.K., Gromov, S.P., Vatsadze, S.Z., Nuriev, V.N., Medvedko, A.V.: Photoprocesses in bis(18-crown-6)-1,3-distyrylbenzene and its complexes with metal perchlorates. Dyes Pigments 184, 108773–108782 (2021). https://doi.org/10.1016/j.dyepig.2020.108773
Acknowledgements
This research was done in frames of project funded by the Russian Science Foundation (Project No. 22-23-00161), except synthesis of (E)-bis(18-crown-6)azobenzene and bis(ammoniopropyl) derivatives of bipyridine and dipyridylethylene. Synthesis of the (E)-bis(18-crown-6)azobenzene and bis(ammoniopropyl) derivatives was done in a framework of project No. 22-13-00064 of the Russian Science Foundation.
Author information
Authors and Affiliations
Contributions
V. Volchkov - steady-state measurements, writing the manuscript, editing M. Khimich, R. Starostin, and A. Freidzon - quantum-chemical calculations, writing the quantum-chemical paragraph A. Egorov - measurements of fluorescence kinetics S. Dmitrieva, R. Starostin - organic synthesis M. Melnikov, S. Gromov - reviewing the manuscript
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Volchkov, V.V., Khimich, M.N., Melnikov, M.Y. et al. Hydrogen-Bonded Self-assembly of Supramolecular Donor–Acceptor Complexes of (E)-Bis(18-crown-6)azobenzene with Bis(ammoniopropyl) Derivatives of Bipyridine and Dipyridylethylene in Acetonitrile. J Solution Chem 52, 805–822 (2023). https://doi.org/10.1007/s10953-023-01271-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-023-01271-6