Abstract
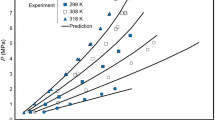
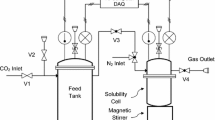
Accurate calculation of light hydrocarbon solubilities in aqueous electrolyte solutions is critical for petroleum and geochemical applications. However, very few electrolyte Equations of State (e-EoS) have been used for calculation of light hydrocarbon solubilities. Thermodynamic calculation of gas (methane and ethane) solubilities in aqueous solutions of chloride salts are presented. This work introduces a new parameter estimation approach (which can predict well the liquid densities of aqueous solutions) for the electrolyte Cubic Plus Association EoS (e-CPA). The cation/anion–water interaction parameters are obtained from the regression of experimental mean ionic activity coefficients and liquid densities. The results show that the e-CPA with the new parameter estimation approach gives good agreements with the corresponding experimental data. The cation/anion–gas interaction parameters are obtained from the regression of experimental gas solubilities. The results show that the e-CPA with the new parameter estimation approach correlates the gas solubilities reasonably well. The gas dissolution mechanisms and salting effects are extensively analyzed and discussed for a deeper understanding of gas dissolution in aqueous electrolyte solutions.










Similar content being viewed by others
References
Zhang, S., Taylor, M.K., Jiang, L., Ren, H., Zhu, G.: Light hydrocarbon separations using porous organic framework materials. Chemistry Eur. J. 26, 3205–3221 (2020)
Makhoukhi, S., Marignac, C., Pironon, J., Schmitt, J., Marrakchi, C., Bouabdelli, M., Bastoul, A.: Aqueous and hydrocarbon inclusions in dolomite from Touissit-Bou Beker district, Eastern Morocco: a Jurassic carbonate hosted PbZn (Cu) deposit. J. Geochem. Explor. 78, 545–551 (2003)
Gnanendran, N., Amin, R.: Equilibrium hydrate formation conditions for hydrotrope–water–natural gas systems. Fluid Phase Equilib. 221, 175–187 (2004)
Nabipour, N., Mosavi, A., Baghban, A., Shamshirband, S., Felde, I.: Extreme learning machine-based model for solubility estimation of hydrocarbon gases in electrolyte solutions. Processes 8, 92 (2020). https://doi.org/10.3390/pr8010092
Harvey, A.H., Prausnitz, J.M.: Thermodynamics of high-pressure aqueous systems containing gases and salts. AIChE J. 35, 635–644 (1989)
Blum, L.: Mean spherical model for asymmetric electrolytes: I. Method of solution. Mol. Phys. 30, 1529–1535 (1975)
Born, M.: Volumen und hydratationswärme der ionen. Z. Phys. 1, 45–48 (1920)
Aasberg-Petersen, K., Stenby, E., Fredenslund, A.: Prediction of high-pressure gas solubilities in aqueous mixtures of electrolytes. Ind. Eng. Chem. Res. 30, 2180–2185 (1991)
Adachi, Y., Lu, B.C.-Y., Sugie, H.: A four-parameter equation of state. Fluid Phase Equilib. 11, 29–48 (1983)
Debye, P., Huckel, E.: Zur Theorie der Elektrolyte. I. Gefrierpunktserniedrigung und verwandte Erscheinungen. Phys. Z. 24, 185–206 (1923)
Haghighi, H., Chapoy, A., Tohidi, B.: Methane and water phase equilibria in the presence of single and mixed electrolyte solutions using the cubic-plus-association equation of state. Oil Gas Sci. Technol. 64, 141–154 (2009)
Kontogeorgis, G.M., Voutsas, E.C., Yakoumis, I.V., Tassios, D.P.: An equation of state for associating fluids. Ind. Eng Chem. Res. 35, 4310–4318 (1996)
Maribo-Mogensen, B., Kontogeorgis, G.M., Thomsen, K.: Comparison of the Debye-Hückel and the mean spherical approximation theories for electrolyte solutions. Ind. Eng. Chem. Res. 51, 5353–5363 (2012)
Peng, D.-Y., Robinson, D.B.: A new two-constant equation of state. Ind. Eng. Chem. Fund. 15, 59–64 (1976)
Søreide, I., Whitson, C.H.: Peng–Robinson predictions for hydrocarbons, CO2, N2, and H2S with pure water and NaCl brine. Fluid Phase Equilib. 77, 217–240 (1992)
Soave, G.: Equilibrium constants from a modified Redlich–Kwong equation of state. Chem. Eng. Sci. 27, 1197–1203 (1972)
Wertheim, M.: Fluids with highly directional attractive forces. I. Statistical thermodynamics. J. Stat. Phys. 35, 19–34 (1984)
Wertheim, M.: Fluids with highly directional attractive forces. II. Thermodynamic perturbation theory and integral equations. J. Stat. Phys. 35, 35–47 (1984)
Michelsen, M.L., Hendriks, E.M.: Physical properties from association models. Fluid Phase Equilib. 180, 165–174 (2001)
Rashin, A.A., Honig, B.: Reevaluation of the Born model of ion hydration. J. Phys. Chem. 89, 5588–5593 (1985)
Stokes, R.H.: Debye model and the primitive model for eectrolyte solutions. J. Chem. Phys. 56, 3382–3383 (1972)
Latimer, W.M., Pitzer, K.S., Slansky, C.M.: The free energy of hydration of gaseous ions, and the absolute potential of the normal calomel electrode. J. Chem. Phys. 7(2), 108–111 (1993)
Maribo-Mogensen, B., Thomsen, K., Kontogeorgis, G.M.: An electrolyte CPA equation of state for mixed solvent electrolytes. AIChE J. 61, 2933–2950 (2015)
Sun, L., Liang, X., Von Solms, N., Kontogeorgis, G.M.: Modeling tetra-n-butyl ammonium halides aqueous solutions with the electrolyte cubic plus association equation of state. Fluid Phase Equilib. 486, 37–47 (2019)
Valiskó, M., Boda, D.: Unraveling the behavior of the individual ionic activity coefficients on the basis of the balance of ion–ion and ion–water interactions. J. Phys. Chem. B 119, 1546–1557 (2015)
Valiskó, M., Boda, D.: Activity coefficients of individual ions in LaCl3 from the II + IW theory. Mol. Phys. 115, 1245–1252 (2017)
Sun, L., Liang, X., von Solms, N., Kontogeorgis, G.M.: Analysis of some electrolyte models including their ability to predict the activity coefficients of individual ions. Ind. Eng. Chem. Res. 59, 11790–11809 (2020)
Marcus, Y.: Ionic radii in aqueous solutions. Chem. Rev. 88, 1475–1498 (1988)
Shannon, R.D.: Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 32, 751–767 (1976)
Rajamani, S., Ghosh, T., Garde, S.: Size dependent ion hydration, its asymmetry, and convergence to macroscopic behavior. J. Chem. Phys. 120, 4457–4466 (2004)
Sun, L., Kontogeorgis, G.M., von Solms, N., Liang, X.: Modeling of gas solubility using the electrolyte cubic plus association equation of state. Ind. Eng. Chem. Res. 58, 17555–17567 (2019)
Sun, L., Liang, X., von Solms, N., Kontogeorgis, G.M.: Thermodynamic modeling of gas solubility in aqueous solutions of quaternary ammonium salts with the e-CPA equation of state. Fluid Phase Equilib. 507, 112423 (2020)
Culberson, O., McKetta, J., Jr.: Phase equilibria in hydrocarbon–water systems. III. The solubility of methane in water at pressures to 10,000 psia. J. Petrol. Technol. 3, 223–226 (1951)
Culberson, O., McKetta, J., Jr.: Phase equilibria in hydrocarbon–water systems. IV. Vapor–liquid equilibrium constants in the methane–water and ethane–water systems. J. Petrol. Technol. 3, 297–300 (1951)
Borina, A., Samojlov, O.Y.: On connection of temperature dependence of neon solubility in aqueous solutions with structural solvent state. Zh. Strukt. Khim. 15, 395–402 (1974)
Jhon, M., Eyring, H., Sung, Y.: Solubility of gases in water. Chem. Phys. Lett. 13, 36–39 (1972)
Kuenen, J.P.: LVIII mixtures of hydrochloric acid and methylether. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1, 593–598 (1901)
Tammann, G.: Die Gaslöslichkeit in Abhängigkeit von der Temperatur. Z. Anorg. Allgem. Chem. 158, 17–24 (1926)
Groisman, A.S., Khomutov, N.: Solubility of oxygen in electrolyte solutions. Russ. Chem. Rev. 59, 707 (1990)
Thomsen, K.: Data Bank for Electrolyte Solutions. Technical University of Denmark, Kgs. Lyngby (2016)
Fawcett, W.R., Tikanen, A.C.: Role of solvent permittivity in estimation of electrolyte activity coefficients on the basis of the mean spherical approximation. J. Phys. Chem. 100, 4251–4255 (1996)
Duan, Z., Møller, N., Greenberg, J., Weare, J.H.: The prediction of methane solubility in natural waters to high ionic strength from 0 to 250 C and from 0 to 1600 bar. Geochim. Cosmochim. Acta 56, 1451–1460 (1992)
Duffy, J.R., Smith, N.O., Nagy, B.: Solubility of natural gases in aqueous salt solutions—I: liquidus surfaces in the system CH4–H2O–NaCl–CaCl2 at room temperatures and at pressures below 1000 psia. Geochim. Cosmochim. Acta 24, 23–31 (1961)
Michels, A., Gerver, J., Bijl, A.: The influence of pressure on the solubility of gases. Physica 3, 797–808 (1936)
Barta, L., Bradley, D.J.: Extension of the specific interaction model to include gas solubilities in high temperature brines. Geochim. Cosmochim. Acta 49, 195–203 (1985)
Blanco, C.L.H., Smith, N.O.: The high pressure solubility of methane in aqueous calcium chloride and aqueous tetraethylammonium bromide. Partial molar properties of dissolved methane and nitrogen in relation to water structure. J. Phys. Chem. 82, 186–191 (1978)
Mao, S., Zhang, Z., Hu, J., Sun, R., Duan, Z.: An accurate model for calculating C2H6 solubility in pure water and aqueous NaCl solutions. Fluid Phase Equilib. 238, 77–86 (2005)
Czerski, L., Czapliński, A.: Solubility of ethane in water and NaCl and CaCl2 solutions at 0.0 C and pressure above 1 atm. Rocz. Chem. Ann. Soc. Chim. Polonorum 36, 1827–1834 (1962)
Mishnina, T., Avdeeva, O., Bozhovskaya, T.: Materialy Vses. Nauchn-Issled Geol. Inst. 46, 93–110 (1961)
Stoessell, R.K., Byrne, P.A.: Salting-out of methane in single-salt solutions at 25 C and below 800 psia. Geochim. Cosmochim. Acta 46, 1327–1332 (1982)
O’Sullivan, T.D., Smith, N.O.: Solubility and partial molar volume of nitrogen and methane in water and in aqueous sodium chloride from 50 to 125° and 100 to 600 atm. J. Phys. Chem. 74, 1460–1466 (1970)
Kiepe, J., Horstmann, S., Fischer, K., Gmehling, J.: Experimental determination and prediction of gas solubility data for methane + water solutions containing different monovalent electrolytes. Ind. Eng. Chem. Res. 42, 5392–5398 (2003)
Eucken, A., Hertzberg, G.: Aussalzeffekt und ionenhydratation. Z. Phys. Chem. 195, 1–23 (1950)
Ben-Naim, A., Yaacobi, M.: Effects of solutes on the strength of hydrophobic interaction and its temperature dependence. J. Phys. Chem. 78, 170–175 (1974)
Wilhelm, E., Battino, R., Wilcock, R.J.: Low-pressure solubility of gases in liquid water. Chem. Rev. 77, 219–262 (1977)
Acknowledgements
The authors thank College of Mechanical and Electrical Engineering at Hohai University, and Department of Chemical and Biochemical Engineering at Technical University of Denmark.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, L., Liang, J. Solubility Calculations of Methane and Ethane in Aqueous Electrolyte Solutions. J Solution Chem 50, 920–940 (2021). https://doi.org/10.1007/s10953-021-01087-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-021-01087-2