Abstract
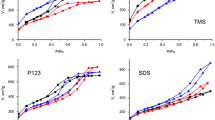
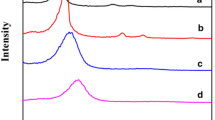
Templated sol–gel synthesis of silica materials with hexagonally ordered mesoporous structure was realized in the presence of amphiphilic organic compounds (azo dyes, bile acid, cyclic oligosaccharide) as cosurfactants and alkoxysilane derivatives prepared on their basis as structure-forming silanes. The effect of auxiliary agents on mesoporous structure of resulting silicas was estimated by low-temperature nitrogen adsorption–desorption and x-ray diffraction analysis. It was found that introduction of moderate amounts of amphiphilic organic additives or their alkoxysilane derivatives in sol–gel reaction mixture causes formation of silica materials with higher surface area and more distinct long-range ordered pore system. Obtained results open up new opportunities for synthesis of MCM-41-type silica materials with improved structural characteristics.











Similar content being viewed by others
References
C.T. Kresge, M.E. Leonowicz, W.J. Roth, J.C. Vartuli, J.S. Beck, Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 359, 710–712 (1992). https://doi.org/10.1038/359710a0
T. Yanagisawa, T. Shimizu, K. Kuroda, C. Kato, The preparation of alkyltriinethylaininonium–kaneinite complexes and their conversion to microporous materials. Bull. Chem. Soc. Jpn. 63, 988–992 (1990). https://doi.org/10.1246/bcsj.63.988
T. Zhang, B. Li, X. Zhang, J. Wang, L. Wei, B. Zhao, B. Li, 4-Dimethylaminopyridine grafted on MCM-41 as an efficient and highly stable catalyst for the production of α-tocopherol acetate. J. Porous Mater. 27, 1639–1648 (2020). https://doi.org/10.1007/s10934-020-00939-4
S. Shirali, A.S. Beni, Preparation and characterization of novel hybrid nanomaterial catalyst MCM-41@AzaCrown-SB-Cu and its application in synthesis of 1,2,3-triazole derivatives in click chemistry. J. Porous Mater. 27, 1601–1611 (2020). https://doi.org/10.1007/s10934-020-00924-x
H. Yoshitake, Highly-controlled synthesis of organic layers on mesoporous silica: their structure and application to toxic ion adsorptions. New J. Chem. 29, 1107–1117 (2005). https://doi.org/10.1039/B504957A
G.E. Fryxell, S.V. Mattigod, Y. Lin, H. Wu, S. Fiskum, K. Parker, F. Zheng, W. Yantasee, T.S. Zemanian, R.S. Addleman, J. Liu, K. Kemner, S. Kelly, X. Feng, Design and synthesis of self-assembled monolayers on mesoporous supports (SAMMS): the importance of ligand posture in functional nanomaterials. J. Mater. Chem. 17, 2863–2874 (2007). https://doi.org/10.1039/B702422C
A. Jomekian, A. Shafiee, A. Moradian, Synthesis of new modified MCM-41/PSF nanocomposite membrane for improvement of water permeation flux. Desalin. Water. Treat. 41, 53–61 (2012). https://doi.org/10.1080/19443994.2012.664678
Y. Bao, X. Yan, W. Du, X. **e, Z. Pan, J. Zhou, L. Li, Application of amine-functionalized MCM-41 modified ultrafiltration membrane to remove chromium (VI) and copper (II). Chem. Eng. J. 28, 460–467 (2015). https://doi.org/10.1016/j.cej.2015.06.094
B.J. Melde, B.J. Johnson, P.T. Charles, Mesoporous silicate materials in sensing. Sensors (Basel) 8, 5202–5228 (2008). https://doi.org/10.3390/s8085202
J.C. Ndamanisha, L. Guo, Ordered mesoporous carbon for electrochemical sensing: a review. Anal. Chim. Acta 747, 19–28 (2012). https://doi.org/10.1016/j.aca.2012.08.032
N.V. Roik, L.A. Belyakova, M.O. Dziazko, Optically transparent silica film with pH-sensing properties: influence of chemical immobilization and presence of β-cyclodextrin on protolytic properties of alizarin yellow. Sensor. Actuat. B. Chem. 273, 1103–1112 (2018)
M. Vallet-Regi, A. Ramila, R.P. del Real, J. Perez-Pariente, A new property of MCM-41: drug delivery system. Chem. Mater. 13, 308–311 (2001). https://doi.org/10.1021/cm0011559
I.I. Slowing, J.L. Vivero-Escoto, C.-W. Wu, V.S.-Y. Lin, Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Delivery Rev. 60, 1278–1288 (2008). https://doi.org/10.1016/j.addr.2008.03.012
L. Gao, J. Sun, Y. Li, Functionalized bimodal carriers for controlled aspirin delivery. J. Solid State Chem. 184, 1909–1914 (2011). https://doi.org/10.1016/j.jssc.2011.05.052
P. Yang, S. Gai, J. Lin, Functionalized mesoporous materials for controlled delivery. Chem. Soc. Rev. 41, 3679–3698 (2012). https://doi.org/10.1039/C2CS15308D
Y. Wang, Q. Zhao, N. Han, L. Bai, J. Li, J. Liu, E. Che, L. Hu, Q. Zhang, T. Jiang, S. Wang, Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed. 11, 313–327 (2015). https://doi.org/10.1016/j.nano.2014.09.014
I. Izquierdo-Barba, L. Ruiz-Gonzalez, J.C. Doadrio, J.M. Gonzalez-Calbet, M. Vallet-Regi, Tissue regeneration: a new property of mesoporous materials. Solid State Sci. 7, 983–989 (2005). https://doi.org/10.1016/j.solidstatesciences.2005.04.003
M. Colilla, M. Manzano, M. Vallet-Regi, Recent advances in ceramic implants as drug delivery systems for biomedical applications. Int. J. Nanomed. 3, 403–414 (2008). https://doi.org/10.2147/ijn.s3548
G.R. Beck Jr., S.-W. Ha, C.E. Camalier, M. Yamaguchi, Y. Li, J.-K. Lee, M.N. Weitzmann, Bioactive silica-based nanoparticles stimulate bone-forming osteoblasts, suppress bone-resorbing osteoclasts, and enhance bone mineral density in vivo. Nanomed. 8, 793–803 (2012). https://doi.org/10.1016/j.nano.2011.11.003
X.S. Zhao, G.Q. (Max) Lu, G.J. Millar, Advances in mesoporous molecular sieve MCM-41, Ind. Eng. Chem. Res. 35 (1996) 2075−2090.
Q. Huo, D.I. Margolese, G.D. Stucky, Surfactant control of phases in the synthesis of mesoporous silica-based materials. Chem. Mater. 8, 1147–1160 (1996). https://doi.org/10.1021/cm960137h
R. Xu, W. Pang, J. Yu, Q. Huo, J. Chen, Chemistry of Zeolites and Related Porous Materials: Synthesis and Structure (Wiley & Sons, Singapore, 2007)
Z.A. AlOthman, A review: fundamental aspects of silicate mesoporous materials. Materials 5, 2874–2902 (2012). https://doi.org/10.3390/ma5122874
Q. Qu, G. Zhou, Y. Ding, S. Feng, Z. Gu, Adjustment of the morphology of MCM-41 silica in basic solution. J. Non-Crystal. Solids 405, 104–115 (2014). https://doi.org/10.1016/j.jnoncrysol.2014.09.012
S.A. Sajjadi, A. Izadbakhsh, K. Niknam, Effect of synthesis conditions on textural properties of silica MCM-41. J. Oil Gas Petrochem. Technol. 3, 59–82 (2016)
A. Jafarzadeh, Sh. Sohrabnezhad, M.A. Zanjanchi, M. Arvand, Fabrication of MCM-41 fibers with well-ordered hexagonal mesostructure controlled in acidic and alkaline media. J. Solid State Chem. 242, 236–242 (2016). https://doi.org/10.1016/j.jssc.2016.07.030
J.Y. Ying, C.P. Mehnert, M.S. Wong, Synthesis and applications of supramolecular-templated mesoporous materials. Angew. Chem. Int. Ed. 38, 56–77 (1999)
N.K. Raman, M.T. Anderson, C.J. Brinker, Template-based approaches to the preparation of amorphous, nanoporous silicas. Chem. Mater. 8, 1682–1701 (1996). https://doi.org/10.1021/cm960138+
F. Testa, L. Pasqua, P. Frontera, R. Aiello, Synthesis of MCM-41 materials in the presence of cetylpyridinium surfactant. Stud. Surf. Sci. Cat. 154, 424–431 (2004). https://doi.org/10.1016/S0167-2991(04)80832-2
M.D. Brankovic, A.R. Zarubica, T.D. Andjelkovic, D.H. Andjelkovi, Mesoporous silica (MCM-41): synthesis/modification, characterization and removal of selected organic micro-pollutants from water. Adv. Technol. 6, 50–57 (2017). https://doi.org/10.5937/savteh1701050b
K.W. Park, J.Y. Kim, H.J. Seo, O.-Y. Kwon, Preparation of mesoporous silica by nonionic surfactant micelle–templated gelation of Na2SiO3 and H2SiF6 and application as a catalyst carrier for the partial oxidation of CH4. Sci. Rep. 9, 13360 (2019). https://doi.org/10.1038/s41598-019-50053-y
J.L. Blin, A. Becue, B. Pauwels, G. Van Tendeloo, B.L. Su, Non-ionic surfactant C13EOm, m=6, 12 and 18) for large poremesoporous molecular sieves preparation. Micropor. Mesopor. Mat. 44–45, 41–51 (2001). https://doi.org/10.1016/S1387-1811(01)00167-6
A. Leonard, J.L. Blin, B.-L. Su, Synthesis of highly ordered mesoporous compounds with control of morphology using a non-ionic surfactant as template. Stud. Surf. Sci. Cat. 141, 109–116 (2002). https://doi.org/10.1016/s0167-2991(02)80531-6
B.J. Pang, K.Y. Qiu, Y. Wei, X.-J. Lei, Z.F. Liu, Facile preparation of transparent and monolithic mesoporous silica materials. Chem. Comm. 6, 477–478 (2000). https://doi.org/10.1039/A909420B
Y. Wei, D. **, T. Ding, W.-H. Shih, X. Liu, S.Z.D. Cheng, Q. Fu, A non-surfactant templating route to mesoporous silica materials. Adv. Mater. 10, 313–316 (1998)
J.-Y. Zheng, J.-B. Pang, K.-Y. Qiu, Y. Wei, Synthesis of mesoporous silica materials via nonsurfactant templated sol–gel route by using mixture of organic compounds as template. J. Sol-Gel Sci. Tech. 24, 81–88 (2002). https://doi.org/10.1023/A:1015117717642
J.S. Beck, J.C. Vartuli, W.J. Roth, M.E. Leonowicz, C.T. Kresge, K.D. Schmitt, C.T.W. Chu, D.H. Olson, E.W. Sheppard, S.B. McCullen, J.B. Higgins, J.L. Schlenker, A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 114, 10834–10843 (1992). https://doi.org/10.1021/ja00053a020
S. Namba, A. Mochizuki, M. Kito, Preparation of highly ordered MCM-41 with docosyltrimethylammonium chloride (C22TMAC1) as a template and fine control of its pore size. Stud. Surf. Sci. Catal. 117, 257–264 (1998). https://doi.org/10.1016/S0167-2991(98)81000-8
S.K. Jana, A. Mochizuki, S. Namba, Progress in pore-size control of mesoporous MCM-41 molecular sieve using surfactant having different alkyl chain lengths and various organic auxiliary chemicals, Catal. Surv. Asia 8, 1–13 (2004). https://doi.org/10.1023/B:CATS.0000015110.85694.d9
B. Alireza, V. Razieh, H. Abed (2008) Study of silanolate groups (≡SiO−) in synthesis of micelle templated silica with various condition of cationic surfactant, Iran. J. Chem. Chem. Eng. 27, 1-6. https://doi.org/10.30492/ijcce.2008.6927.
C. Liu, X. Wang, S. Lee, L.D. Pfefferle, G.L. Haller, Surfactant chain length effect on the hexagonal-to-cubic phase transition in mesoporous silica synthesis. Micropor. Mesopor. Mater. 147, 242–251 (2012). https://doi.org/10.1016/j.micromeso.2011.06.021
A.-M. Putz, S. Cecilia, C. Ianaşi, Z. Dudas, K.N. Szekely, J. Plocek, P. Sfarloaga, L. Sacarescu, L. Almasy, Pore ordering in mesoporous matrices induced by different directing agents. J. Porous Mater. 22, 321–331 (2015). https://doi.org/10.1007/s10934-014-9899-z
N. Ulagappan, C.N.R. Rao, Evidence for supramolecular organization of alkane and surfactant molecules in the process of forming mesoporous silica. Chem. Commun. 24, 2759–2760 (1996). https://doi.org/10.1039/CC9960002759
J.L. Blin, B.L. Su, Tailoring pore size of ordered mesoporous silicas using one or two organic auxiliaries as expanders. Langmuir 18, 5303–5308 (2002). https://doi.org/10.1021/la020042w
H. Zhang, X. Li, Novel mesoporous silica materials with hierarchically ordered nanochannel: synthesis with the assistance of straight-chain alkanes and application. J. Chem. 7, 1–16 (2016). https://doi.org/10.1155/2016/5146573
E. Junquera, E. Aicart, G. Tardajos, Inclusional complexes of decyltrimethylammonium bromide and β-cyclodextrin in water. J. Phys. Chem. 96, 4533–4537 (1992). https://doi.org/10.1021/j100190a074
W.M.Z. Wan Yunus, J. Taylor, D.M. Bloor, D.G. Hall, E. Wyn-Jones, Electrochemlcal measurements on the binding of sodium dodecyl sulfate and dodecyltrimethylammonium bromide with α- and β-cyciodextrins, J. Phys. Chem. 96 (1992) 8979−8982. https://doi.org/10.1021/j100201a052.
Y.-B. Jiang, X.-J. Wang, Direct evidence for β-cyclodextrin-induced aggregation of ionic surfactant below critical micelle concentration. Appl. Spectr. 48, 1428–1431 (1994)
A.A. Rafati, A. Bagheri, H. Iloukhani, M. Zarinehzad, Study of inclusion complex formation between a homologous series of n-alkyltrimethylammonium bromides and β-cyclodextrin, using conductometric technique. J. Mol. Liq. 116, 37–41 (2005). https://doi.org/10.1016/j.molliq.2004.05.003
Y.M. Cho, W.K. Lee, B.-K. Kim, Studies on the interaction of azo dyes with cationic surfactant (I). Arch. Pharm. Res. 4, 75–84 (1981). https://doi.org/10.1007/BF02855749
V.C. Reinsborough, J.F. Holzwart, Kinetics of the interactions between dyes and micelles. Can. J. Chem. 64, 955–959 (1986). https://doi.org/10.1139/v86-159
M.F. Nazar, S.S. Shah, M.A. Khosa, Interaction of azo dye with cationic surfactant under different pH conditions. J. Surfactants Deterg. 13, 529–537 (2010). https://doi.org/10.1007/s11743-009-1177-8
M. Swanson-Vethamuthu, M. Almgren, P. Hansson, J. Zhao, Surface tension studies of cetyltrimethylammonium bromide−bile salt association. Langmuir 12, 2186–2189 (1996). https://doi.org/10.1021/la950856v
C. Rottman, A. Turniansky, D. Avnir, Sol-gel physical and covalent entrapment of three methyl red indicators: a comparative study. J. Sol-Gel Sci. Technol. 13, 17–25 (1998). https://doi.org/10.1023/A:1008630701220
L. Travaglini, P. Picchetti, A. Del Giudice, L. Galantini, L. De Cola, Tuning and controlling the shape of mesoporous silica particles with CTAB/sodium deoxycholate catanionic mixtures. Micropor. Mesopor. Mater. 279, 423–431 (2019). https://doi.org/10.1016/j.micromeso.2019.01.030
S. Brunauer, P.H. Emmet, E. Teller, Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 60, 309–319 (1938). https://doi.org/10.1021/ja01269a023
A.V. Neimark, P.I. Ravikovitch, M. Grun, F. Schuth, K.K. Unger, Pore size analysis of MCM-41 type adsorbents by means of nitrogen and argon adsorption. J. Coll. Int. Sci. 207, 159–169 (1998). https://doi.org/10.1006/jcis.1998.5748
S.H. Gregg, K.S. Sing, Adsorption, Surface Area and Porosity (Academic Press, New York, 1967)
W.L. Bragg, The diffraction of short electromagnetic waves by a crystal. P. Camb. Philos. Soc 17, 43–57 (1913)
V.B. Fenelonov, V.N. Romannikov, AYu. Derevyankin, Mesopore size and surface area calculations for hexagonal mesophases (types MCM-41, FSM-16, etc.) using low-angle XRD and adsorption data. Micropor. Mesopor. Mater. 28, 57–72 (1999). https://doi.org/10.1016/S1387-1811(98)00280-7
M. Grun, K.K. Unger, A. Matsumoto, K. Tsutsumi, Novel pathways for the preparation of mesoporous MCM-41 materials: control of porosity and morphology. Micropor. Mesopor. Mater. 27, 207–216 (1999). https://doi.org/10.1016/S1387-1811(98)00255-8
P. Van Der Voort, P.I. Ravikovitch, K.P. De Jong, M. Benjelloun, E. Van Bavel, A.H. Janssen, A.V. Neimark, B.M. Weckhuysen, E.F. Vansant, A new templated ordered structure with combined micro- and mesopores and internal silica nanocapsules. J. Phys. Chem. B 106, 5873–5877 (2002). https://doi.org/10.1021/jp025642i
M. Thommes, B. Smarsly, M. Groenewolt, P.I. Ravikovitch, A.V. Neimark, Adsorption hysteresis of nitrogen and argon in pore networks and characterization of novel micro- and mesoporous silicas. Langmuir 22, 756–764 (2006). https://doi.org/10.1021/la051686h
W. Lai, S. Yang, Y. Jiang, F. Zhao, Z. Li, B. Zaman, M. Fayaz, X. Li, Y. Chen, Artefact peaks of pore size distributions caused by unclosed sorption isotherm and tensile strength effect. Adsorption 26, 633–644 (2020). https://doi.org/10.1007/s10450-020-00228-1
J. Landers, GYu. Gor, A.V. Neimark, Density functional theory methods for characterization of porous materials. Colloid. Surface. A 437, 3–32 (2013). https://doi.org/10.1016/j.colsurfa.2013.01.007
Funding
We received no funding for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Roik, N.V., Dziazko, M.O., Trofymchuk, I.M. et al. Role of amphiphilic organic additives in design of silica materials with ordered mesoporous structure. J Porous Mater 29, 317–330 (2022). https://doi.org/10.1007/s10934-021-01167-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-021-01167-0