Abstract
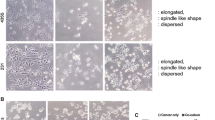
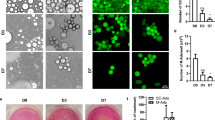
Cancer metastases are accountable for almost 90% of all human cancer related deaths including from breast cancer (BC). Adipocytes can alter the tumor microenvironment, which can promote metastasis by inducing an epithelial-to-mesenchymal transition (EMT) in BC cells. However, the role of adipocytes during the mesenchymal-to-epithelial transition (MET), that can be important in metastasis, is not clear. To understand the effect of adipocytes on the BC progression, there is a requirement for a better in vitro 3-dimensional (3D) co-culture system that mimics the breast tissue and allows for more accurate analysis of EMT and MET. We developed a co-culture system to analyze the relationship of BC cells grown in a 3D culture with adipocytes. We found that adipocytes and adipocyte-derived conditioned media, but not pre-adipocytes, caused the mesenchymal MDA-MB-231 and Hs578t cells to form significantly more epithelial-like structures when compared to the typical stellate colonies formed in control 3D cultures. SUM159 cells and MCF7 cells had a less dramatic shift as they normally have more epithelial-like structure in 3D culture. Biomarker expression analysis revealed that adipocytes only induced a partial MET with proliferation unaffected. In addition, adipocytes had reduced lipid droplet size when co-cultured with BC cells. Thus, we found that physical interaction with adipocytes and ECM changes the mesenchymal phenotype of BC cells in a manner that could promote secondary tumor formation.







Similar content being viewed by others
References
Canadian Cancer Statistics 2017. Canadian Cancer Society’s Advisory Committee on cancer statistics. Can Cancer Soc. 2017. https://cancer.ca/canadian-cancer-statistics-2017-EN.pdf. Accessed 3 March 2018.
** X, Mu P. Targeting breast cancer metastasis. Breast Cancer (Auckl). 2015;9:23–34. https://doi.org/10.4137/BCBCR.S25460.
Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–64. https://doi.org/10.1126/science.1203543.
Lee Y, Jung WH, Koo JS. Adipocytes can induce epithelial-mesenchymal transition in breast cancer cells. Breast Cancer Res Treat. 2015;153:323–35. https://doi.org/10.1007/s10549-015-3550-9.
Ritter A, Friemel A, Fornoff F, Adjan M, Solbach C, Yuan J, et al. Characterization of adipose-derived stem cells from subcutaneous and visceral adipose tissues and their function in breast cancer cells. Oncotarget. 2015;6:34475–93. https://doi.org/10.18632/oncotarget.5922.
Place AE, ** Huh S, Polyak K. The microenvironment in breast cancer progression: biology and implications for treatment. Breast Cancer Res. 2011;13:227. https://doi.org/10.1186/bcr2912.
Howlett AR, Bissell MJ. The influence of tissue microenvironment (stroma and extracellular matrix) on the development and function of mammary epithelium. Epithelial Cell Biol. 1993;2:79–89.
Chaudhuri O, Koshy ST, Branco da Cunha C, Shin JW, Verbeke CS, Allison KH, et al. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat Mater. 2014;13:970–8. https://doi.org/10.1038/nmat4009.
Pellegrinelli V, Heuvingh J, du Roure O, Rouault C, Devulder A, Klein C, et al. Human adipocyte function is impacted by mechanical cues. J Pathol. 2014;233:183–95. https://doi.org/10.1002/path.4347.
Seo BR, Bhardwaj P, Choi S, Gonzalez J, Andresen Eguiluz RC, Wang K, et al. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci Transl Med. 2015;7:301ra130. https://doi.org/10.1126/scitranslmed.3010467.
Duval K, Grover H, Han L-H, Mou Y, Pegoraro AF, Fredberg J, et al. Modeling physiological events in 2D vs. 3D cell culture. Physiology (Bethesda). 2017;32:266–77. https://doi.org/10.1152/physiol.00036.2016.
Kenny PA, Lee GY, Myers CA, Neve RM, Semeiks JR, Spellman PT, et al. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol. 2007;1:84–96. https://doi.org/10.1016/j.molonc.2007.02.004.
Ravi M, Paramesh V, Kaviya SR, Anuradha E, Solomon FDP. 3D cell culture systems: advantages and applications. J Cell Physiol. 2015;230:16–26. https://doi.org/10.1002/jcp.24683.
Baker BM, Chen CS. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125:3015–24. https://doi.org/10.1242/jcs.079509.
Luca AC, Mersch S, Deenen R, Schmidt S, Messner I, Schäfer KL, et al. Impact of the 3D microenvironment on phenotype, gene expression, and EGFR inhibition of colorectal cancer cell lines. PLoS One. 2013;8:e59689. https://doi.org/10.1371/journal.pone.0059689.
Aljitawi OS, Li D, **ao Y, Zhang D, Ramachandran K, Stehno-Bittel L, et al. A novel three-dimensional stromal-based model for in vitro chemotherapy sensitivity testing of leukemia cells. Leuk Lymphoma. 2014;55:378–91. https://doi.org/10.3109/10428194.2013.793323.
Fang Y, Eglen RM. Three-dimensional cell cultures in drug discovery and development. SLAS Discov. 2017;22:456–72. https://doi.org/10.1177/1087057117696795.
Bidarra SJ, Oliveira P, Rocha S, Saraiva DP, Oliveira C, Barrias CC. A 3D in vitro model to explore the inter-conversion between epithelial and mesenchymal states during EMT and its reversion. Sci Rep. 2016;6:27072. https://doi.org/10.1038/srep27072.
Salameh TS, Le TT, Nichols MB, et al. An ex vivo co-culture model system to evaluate stromal-epithelial interactions in breast cancer. Int J Cancer. 2013;132:288–96. https://doi.org/10.1002/ijc.27672.
Herroon MK, Diedrich JD, Podgorski I. New 3D-culture approaches to study interactions of bone marrow adipocytes with metastatic prostate cancer cells. Front Endocrinol (Lausanne). 2016;7:84. https://doi.org/10.3389/fendo.2016.00084.
Kimlin LC, Casagrande G, Virador VM. In vitro three-dimensional (3D) models in cancer research: an update. Mol Carcinog. 2013;52:167–82. https://doi.org/10.1002/mc.21844.
Huang J, Duran A, Reina-Campos M, Valencia T, Castilla EA, Müller TD, et al. Adipocyte p62/SQSTM1 suppresses tumorigenesis through opposite regulations of metabolism in adipose tissue and tumor. Cancer Cell. 2018;33:770–784.e6. https://doi.org/10.1016/j.ccell.2018.03.001.
Smith NC, Fairbridge NA, Pallegar NK, Christian SL. Dynamic upregulation of CD24 in pre-adipocytes promotes adipogenesis. Adipocyte. 2014;4:89–100. https://doi.org/10.4161/21623945.2014.985015.
Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–68.
Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5.
Grishagin IV. Automatic cell counting with ImageJ. Anal Biochem. 2015;473:63-5. https://doi.org/10.1016/j.ab.2014.12.007.
R Core Development Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2015.
RStudio Team. RStudio: integrated development for R. Boston: RStudio, Inc.; 2015.
Barnabas N, Cohen D. Phenotypic and molecular characterization of MCF10DCIS and SUM breast cancer cell lines. Int J Breast Cancer. 2013;2013:1–16. https://doi.org/10.1155/2013/872743.
Lee GY, Kenny PA, Lee EH, Bissell MJ. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods. 2007;4:359–65. https://doi.org/10.1038/nmeth1015.
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96. https://doi.org/10.1038/nrm3758.
Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–67. https://doi.org/10.1172/JCI45014.
Xu K, Buchsbaum RJ. Isolation of mammary epithelial cells from three-dimensional mixed-cell spheroid co-culture. J Vis Exp. 2012. https://doi.org/10.3791/3760.
Xu K, Tian X, Oh SY, Movassaghi M, Naber SP, Kuperwasser C, et al. The fibroblast Tiam1-osteopontin pathway modulates breast cancer invasion and metastasis. Breast Cancer Res. 2016;18(14):14. https://doi.org/10.1186/s13058-016-0674-8.
Sawant S, Dongre H, Singh AK, Joshi S, Costea DE, Mahadik S, et al. Establishment of 3D co-culture models from different stages of human tongue tumorigenesis: utility in understanding neoplastic progression. PLoS One. 2016;11:e0160615. https://doi.org/10.1371/journal.pone.0160615.
Sundaram S, Johnson AR, Makowski L. Obesity, metabolism and the microenvironment: links to cancer. J Carcinog. 2013;12:19. https://doi.org/10.4103/1477-3163.119606.
Chkourko Gusky H, Diedrich J, MacDougald OA, Podgorski I. Omentum and bone marrow: how adipocyte-rich organs create tumour microenvironments conducive for metastatic progression. Obes Rev. 2016;17:1015–29. https://doi.org/10.1111/obr.12450.
Hardaway AL, Herroon MK, Rajagurubandara E, Podgorski I. Bone marrow fat: linking adipocyte-induced inflammation with skeletal metastases. Cancer Metastasis Rev. 2014;33:527–43. https://doi.org/10.1007/s10555-013-9484-y.
Hilvo M, Orešič M. Regulation of lipid metabolism in breast cancer provides diagnostic and therapeutic opportunities. Clin Lipidol. 2012;7:177–88. https://doi.org/10.2217/clp.12.10.
Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–503. https://doi.org/10.1038/nm.2492.
Pierobon M, Frankenfeld CL. Obesity as a risk factor for triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;137:307–14. https://doi.org/10.1007/s10549-012-2339-3.
Simpson KJ, Dugan AS, Mercurio AM. Functional analysis of the contribution of RhoA and RhoC GTPases to invasive breast carcinoma. Cancer Res. 2004;64:8694–701. https://doi.org/10.1158/0008-5472.CAN-04-2247.
Funding
Funding provided to SLC by the Memorial University of Newfoundland (210525), a New Investigator Award from the Beatrice Hunter Cancer Research Institute (207939), and the Cancer Research Society (22130). An NSERC Discovery Grant to AVP provided funding for optimization of the 3D culture model (RGPIN-2017-3977). NKP was supported by a trainee award and a Skills Acquisition Program award from the Beatrice Hunter Cancer Research Institute with funds provided by The Terry Fox Strategic Health Research Training Program in Cancer Research at CIHR and by Memorial University of Newfoundland.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Approval
This article does not contain any studies with human participants or animals.
Rights and permissions
About this article
Cite this article
Pallegar, N.K., Garland, C.J., Mahendralingam, M. et al. A Novel 3-Dimensional Co-culture Method Reveals a Partial Mesenchymal to Epithelial Transition in Breast Cancer Cells Induced by Adipocytes. J Mammary Gland Biol Neoplasia 24, 85–97 (2019). https://doi.org/10.1007/s10911-018-9420-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10911-018-9420-4