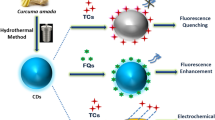
Abstract
The utilization of fluorescent quantum dots (FL QDs) has gained significant traction in the realm of antibiotic detection, owing to their exceptional FL properties and versatility. Various types of QDs have been tailored to exhibit superior FL characteristics, employing diverse cap** agents such as metals, surfactants, polymers, and biomass to protect and stabilize their surfaces. In their evolution, FL QDs have demonstrated both “turn-off” and “turn-on” mechanisms in response to the presence of analytes, offering promising avenues for biosensing applications. This review article provides a comprehensive overview of the recent advancements in antibiotic detection utilizing FL QDs as biosensors. It encompasses an extensive examination of different types of FL QDs, including carbon, metal, and core-shell QDs, deployed for the detection of antibiotics. Furthermore, the synthesis methods employed for the fabrication of various FL QDs are elucidated, shedding light on the diverse approaches adopted in their preparation. Moreover, this review delves into the intricate sensing mechanisms underlying FL QDs-based antibiotic detection. Various mechanisms, such as photoinduced electron transfer, electron transfer, charge transfer, Forster resonance energy transfer, static quenching, dynamic quenching, inner filter effect, hydrogen bonding, and aggregation-induced emission, are discussed in detail. These mechanisms provide a robust scientific rationale for the detection of antibiotics using FL QDs, showcasing their potential for sensitive and selective sensing applications. Finally, the review addresses current challenges and offers perspectives on the future improvement of FL QDs in sensing applications. Insights into overcoming existing limitations and harnessing emerging technologies are provided, charting a course for the continued advancement of FL QDs-based biosensing platforms in the field of antibiotic detection.






(Reprinted with permission from Elsevier)

(Reprinted with permission from Elsevier)

(Reprinted with permission from Elsevier)


Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
Tarannum N, Khatoon S, Dzantiev BB (2020) Perspective and application of molecular imprinting approach for antibiotic detection in food and environmental samples: a critical review. Food Control 118:107381. https://doi.org/10.1016/j.foodcont.2020.107381
Wang Q, Zhao WM (2018) Optical methods of antibiotic residues detections: a comprehensive review. Sens Actuators B Chem 269:238–256. https://doi.org/10.1016/j.snb.2018.04.097
Joshi A, Kim KH (2020) Recent advances in nanomaterial-based electrochemical detection of antibiotics: challenges and future perspectives. Biosens Bioelectron 153:112046. https://doi.org/10.1016/j.bios.2020.112046
Yang Y, Yin S, Li Y et al (2017) Application of aptamers in detection and chromatographic purification of antibiotics in different matrices. TrAC - Trends Anal Chem 95:1–22. https://doi.org/10.1016/j.trac.2017.07.023
Shao S, Hu Y, Cheng J, Chen Y (2018) Research progress on distribution, migration, transformation of antibiotics and antibiotic resistance genes (ARGs) in aquatic environment. Crit Rev Biotechnol 38:1195–1208. https://doi.org/10.1080/07388551.2018.1471038
Prestinaci F, Pezzotti P, Pantosti A (2015) Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health 109:309–318. https://doi.org/10.1179/2047773215Y.0000000030
Miller EL (2002) The penicillins: a review and update. J Midwifery Womens Health 47:426–434. https://doi.org/10.1016/S1526-9523(02)00330-6
Tandale P, Choudhary N, Singh J et al (2021) Fluorescent quantum dots: an insight on synthesis and potential biological application as drug carrier in cancer. Biochem Biophys Rep 26:100962. https://doi.org/10.1016/j.bbrep.2021.100962
Jahangir MA, Gilani SJ, Muheem A et al (2019) Quantum dots: Next Generation of Smart Nano-Systems. Pharm Nanotechnol 7:234–245. https://doi.org/10.2174/2211738507666190429113906
Zhou JW, Zou XM, Song SH, Chen GH (2018) Quantum dots Applied to Methodology on detection of Pesticide and Veterinary Drug residues. J Agric Food Chem 66:1307–1319. https://doi.org/10.1021/acs.jafc.7b05119
Wu T, Zhang T, Chen Y, Tang M (2016) Research advances on potential neurotoxicity of quantum dots. J Appl Toxicol 36:345–351. https://doi.org/10.1002/jat.3229
Zhong L, Zhang L, Li Y et al (2021) Assessment of the toxicity of quantum dots through biliometric analysis. Int J Environ Res Public Health 18:5768. https://doi.org/10.3390/ijerph18115768
Yan ZY, Du QQ, Qian J et al (2017) Eco-friendly intracellular biosynthesis of CdS quantum dots without changing Escherichia coli’s antibiotic resistance. Enzyme Microb Technol 96:96–102. https://doi.org/10.1016/j.enzmictec.2016.09.017
Foubert A, Beloglazova NV, De Saeger S (2017) Comparative study of colloidal gold and quantum dots as labels for multiplex screening tests for multi-mycotoxin detection. Anal Chim Acta 955:48–57. https://doi.org/10.1016/j.aca.2016.11.042
Song F, Tang PS, Durst H et al (2012) Nonblinking plasmonic quantum dot assemblies for multiplex biological detection. Angewandte Chemie - Int Ed 51:8773–8777. https://doi.org/10.1002/anie.201201872
Liu X, Wang T, Lu Y et al (2019) Constructing carbon dots and CdTe quantum dots multi-functional composites for ultrasensitive sensing and rapid degrading ciprofloxacin. Sens Actuators B Chem 289:242–251. https://doi.org/10.1016/j.snb.2019.03.094
Yola ML, Atar N (2017) A highly efficient nanomaterial with Molecular Imprinting Polymer: Carbon Nitride nanotubes decorated with Graphene Quantum Dots for Sensitive Electrochemical Determination of Chlorpyrifos. J Electrochem Soc 164:B223–B229. https://doi.org/10.1149/2.1411706jes
Tang J, Wu L, Lin J et al (2021) Development of quantum dot-based fluorescence lateral flow immunoassay strip for rapid and quantitative detection of serum interleukin-6. J Clin Lab Anal 35:e23752. https://doi.org/10.1002/jcla.23752
Yola ML, Atar N (2019) Development of cardiac troponin-I biosensor based on boron nitride quantum dots including molecularly imprinted polymer. Biosens Bioelectron 126:418–424. https://doi.org/10.1016/j.bios.2018.11.016
Wei X, Xu G, Gong C, Qin F, Gong X, Li C (2018) Fabrication and evaluation of sulfanilamide-imprinted composite sensors by develo** a custom-tailored strategy. Sensors and Actuators B: Chemical 255:2697–2703. https://doi.org/10.1016/j.snb.2017.09.081
Akyıldırım O, Kardaş F, Beytur M et al (2017) Palladium nanoparticles functionalized graphene quantum dots with molecularly imprinted polymer for electrochemical analysis of citrinin. J Mol Liq 243:677–681. https://doi.org/10.1016/j.molliq.2017.08.085
Hong GL, Deng HH, Zhao HL et al (2020) Gold nanoclusters/graphene quantum dots complex-based dual-emitting ratiometric fluorescence probe for the determination of glucose. J Pharm Biomed Anal 189:113480. https://doi.org/10.1016/j.jpba.2020.113480
Kang C, Huang Y, Yang H et al (2020) A review of carbon dots produced from biomass wastes. Nanomaterials 10:1–24
Kashani HM, Madrakian T, Afkhami A et al (2019) Bottom-up and green-synthesis route of amino functionalized graphene quantum dot as a novel biocompatible and label-free fluorescence probe for in vitro cellular imaging of human ACHN cell lines. Mater Sci Engineering: B. https://doi.org/10.1016/j.mseb.2019.114452. 251:
Hou S, Da, Zhou SL, Zhang SM, Li HG (2021) Carbon-dot-based solid-state luminescent materials: synthesis and applications in white light emitting diodes and optical sensors. **nxing Tan Cailiao/New Carbon Mater 36:527–545
Yin H, Truskewycz A, Cole IS (2020) Quantum dot (QD)-based probes for multiplexed determination of heavy metal ions. Microchim Acta 187:336. https://doi.org/10.1007/s00604-020-04297-5
Cui XX, Fan Q, Shi SJ et al (2018) A novel near-infrared nanomaterial with high quantum efficiency and its applications in real time in vivo imaging. Nanotechnology 29:205705. https://doi.org/10.1088/1361-6528/aab2fa
Yu WW, Chang E, Drezek R, Colvin VL (2006) Water-soluble quantum dots for biomedical applications. Biochem Biophys Res Commun 348:781–786. https://doi.org/10.1016/j.bbrc.2006.07.160
** L, Wang Y, Liu F et al (2018) The determination of nitrite by a graphene quantum dot fluorescence quenching method without sample pretreatment. Luminescence 33:289–296. https://doi.org/10.1002/bio.3412
Hu Q, Pan Y, Gong X et al (2020) A sensitivity enhanced fluorescence method for the detection of ferrocyanide ions in foodstuffs using carbon nanoparticles as sensing agents. Food Chem 308:125590. https://doi.org/10.1016/j.foodchem.2019.125590
Sahoo H (2011) Förster resonance energy transfer - A spectroscopic nanoruler: Principle and applications. J Photochem Photobiol C 12:20–30. https://doi.org/10.1016/j.jphotochemrev.2011.05.001
Clegg RM (1995) Fluorescence resonance energy transfer. Curr Opin Biotechnol 6:103–113
Sekar RB, Periasamy A (2003) Fluorescence resonance energy transfer (FRET) microscopy imaging of live cell protein localizations. J Cell Biol 160:629–633. https://doi.org/10.1083/jcb.200210140
Zhang X, Hu Y, Yang X et al (2019) FÖrster resonance energy transfer (FRET)-based biosensors for biological applications. Biosens Bioelectron 138:111314. https://doi.org/10.1016/j.bios.2019.05.019
Dale RE, Eisinger J, Blumberg JE (1979) The Orientational Freedom of Molecular Probes. The orientation factor in Intramolecular Energy Transfer. Biophys Soc 26:161–194
Ahmadpour H, Hosseini SMM (2019) A solid-phase luminescence sensor based on molecularly imprinted polymer-CdSeS/ZnS quantum dots for selective extraction and detection of sulfasalazine in biological samples. Talanta 194:534–541. https://doi.org/10.1016/j.talanta.2018.10.053
Fu Y, Huang L, Zhao S et al (2021) A carbon dot-based fluorometric probe for oxytetracycline detection utilizing a Förster resonance energy transfer mechanism. Spectrochim Acta Mol Biomol Spectrosc 246:118947. https://doi.org/10.1016/j.saa.2020.118947
Chen CX, Li YH, Zhou YL et al (2020) Rapidly detecting antibiotics with magnetic nanoparticle coated CdTe quantum dots. RSC Adv 10:1966–1970. https://doi.org/10.1039/c9ra09894a
Gao R, Wu Z, Wang L et al (2020) Green preparation of fluorescent nitrogen-doped carbon quantum dots for sensitive detection of oxytetracycline in environmental samples. Nanomaterials 10:1561. https://doi.org/10.3390/nano10081561
**a Z, Li Q (2020) Application of Metronidazole detection by antibiotic ampicillin sodium based-carbon quantum dots. Int J Environ Anal Chem 102:4178–4190. https://doi.org/10.1080/03067319.2020.1780224
Wang X, Li L, Jiang H et al (2022) Highly selective and sensitive fluorescence detection of tetracyclines based on novel tungsten oxide quantum dots. Food Chem 374:131774. https://doi.org/10.1016/j.foodchem.2021.131774
The Huy B, Thangadurai DT, Sharipov M et al (2022) Recent advances in turn off-on fluorescence sensing strategies for sensitive biochemical analysis - A mechanistic approach. Microchem J 179:107511
Wang X, Zhang L, Hao A et al (2020) Silica-coated silver nanoparticles decorated with fluorescent CdTe quantum dots and DNA aptamers for detection of tetracycline. ACS Appl Nano Mater 3:9796–9803. https://doi.org/10.1021/acsanm.0c01890
Reshma VG, Mohanan PV (2019) Quantum dots: applications and safety consequences. J Lumin 205:287–298. https://doi.org/10.1016/j.jlumin.2018.09.015
Badıllı U, Mollarasouli F, Bakirhan NK et al (2020) Role of quantum dots in pharmaceutical and biomedical analysis, and its application in drug delivery. TrAC - Trends in Analytical Chemistry 131
Cardoso Dos Santos M, Algar WR, Medintz IL, Hildebrandt N (2020) Quantum dots for Förster Resonance Energy Transfer (FRET). TrAC - Trends in Analytical Chemistry 125
Li C, Chen W, Wu D et al (2015) Large Stokes Shift and High Efficiency Luminescent Solar Concentrator Incorporated with CuInS 2 /ZnS Quantum dots. Sci Rep 5. https://doi.org/10.1038/srep17777
Jablonski A (1933) Efficiency of anti-stokes fluorescence in dyes. Nature 839–840
da Luz de Sousa I, **menes VF, de Souza AR, Morgon NH (2019) Solvent-induced Stokes’ shift in DCJTB: experimental and theoretical results. J Mol Struct 1192:186–191. https://doi.org/10.1016/j.molstruc.2019.04.117
Holzapfel HY, Stern AD, Bouhaddou M et al (2018) Fluorescence Multiplexing with Spectral Imaging and Combinatorics. ACS Comb Sci 20:653–659. https://doi.org/10.1021/acscombsci.8b00101
Yang L, Zeng J, Quan T et al (2021) Liquid-liquid extraction and purification of oil red O derived nitrogen-doped highly photoluminescent carbon dots and their application as multi-functional sensing platform for Cu2 + and tetracycline antibiotics. Microchem J 168:106391. https://doi.org/10.1016/j.microc.2021.106391
Zuo G, **e A, Pan X et al (2018) Fluorine-doped Cationic Carbon dots for efficient gene delivery. ACS Appl Nano Mater 1:2376–2385. https://doi.org/10.1021/acsanm.8b00521
Karakoçak BB, Liang J, Kavadiya S et al (2018) Optimizing the synthesis of red-emissive nitrogen-doped carbon dots for use in bioimaging. ACS Appl Nano Mater 1:3682–3692. https://doi.org/10.1021/acsanm.8b00799
Nemati F, Hosseini M, Zare-Dorabei R, Ganjali MR (2018) Sensitive recognition of ethion in food samples using turn-on fluorescence N and S co-doped graphene quantum dots. Anal Methods 10:1760–1766. https://doi.org/10.1039/c7ay02850d
Chaowana R, Bunkoed O (2019) A nanocomposite probe of polydopamine/molecularly imprinted polymer/quantum dots for trace sarafloxacin detection in chicken meat. Anal Bioanal Chem 411:6081–6090. https://doi.org/10.1007/s00216-019-01993-x
Rhodes AA, Swartz BL, Hosler ER et al (2014) Static quenching of tryptophan fluorescence in proteins by a dioxomolybdenum(VI) thiolate complex. J Photochem Photobiol Chem 293:81–87. https://doi.org/10.1016/j.jphotochem.2014.07.023
Crouse HF, Petrunak EM, Donovan AM et al (2011) Static and dynamic quenching of tryptophan fluorescence in various proteins by a chromium (III) complex. Spectrosc Lett 44:369–374. https://doi.org/10.1080/00387010.2010.546470
Rehman MT, Ahmed S, Khan AU (2016) Interaction of meropenem with ‘N’ and ‘B’ isoforms of human serum albumin: a spectroscopic and molecular docking study. J Biomol Struct Dyn 34:1849–1864. https://doi.org/10.1080/07391102.2015.1094411
Hou M, Yan X, **ong L (2015) Determination of sparfloxacin with CdSe/CdS quantum dots as fluorescent probes. J Lumin 157:58–62. https://doi.org/10.1016/j.jlumin.2014.08.006
Liu Z, Hou J, Wang X et al (2020) A novel fluorescence probe for rapid and sensitive detection of tetracyclines residues based on silicon quantum dots. Spectrochim Acta Mol Biomol Spectrosc 240:118463. https://doi.org/10.1016/j.saa.2020.118463
Tsai H, Hu HC, Hsieh CC et al (2020) Fluorescence studies of the interaction between chloramphenicol and nitrogen-doped graphene quantum dots and determination of chloramphenicol in chicken feed. J Chin Chem Soc 67:152–159. https://doi.org/10.1002/jccs.201900124
Xu X, Yang Y, ** H et al (2020) Fungal in situ Assembly gives Novel Properties to CdSxSe1- x Quantum dots for sensitive label-free detection of Chloramphenicol. ACS Sustain Chem Eng 8:6806–6814. https://doi.org/10.1021/acssuschemeng.0c01698
Raksawong P, Chullasat K, Nurerk P et al (2017) A hybrid molecularly imprinted polymer coated quantum dot nanocomposite optosensor for highly sensitive and selective determination of salbutamol in animal feeds and meat samples. Anal Bioanal Chem 409:4697–4707. https://doi.org/10.1007/s00216-017-0466-8
Wang Q, Li L, Wu T et al (2020) A graphene quantum dots-Pb2 + based fluorescent switch for selective and sensitive determination of D-penicillamine. Spectrochim Acta Mol Biomol Spectrosc 229. https://doi.org/10.1016/j.saa.2019.117924
Chen X, Luan Y, Wang N et al (2018) Ratiometric fluorescence nanosensors based on core-shell structured carbon/CdTe quantum dots and surface molecularly imprinted polymers for the detection of sulfadiazine. J Sep Sci 41:4394–4401. https://doi.org/10.1002/jssc.201800866
Shang L, Dong S (2009) Design of fluorescent assays for cyanide and hydrogen peroxide based on the inner filter effect of metal nanoparticles. Anal Chem 81:1465–1470. https://doi.org/10.1021/ac802281x
Kumar Panigrahi S, Kumar Mishra A (2019) Inner filter effect in fluorescence spectroscopy: As a problem and as a solution. Journal of Photochemistry and Photobiology C: Photochemistry Reviews 41
Han L, Fan YZ, Qing M et al (2020) Smartphones and Test Paper-assisted Ratiometric fluorescent sensors for semi-quantitative and visual assay of Tetracycline based on the Target-Induced Synergistic Effect of Antenna Effect and Inner Filter Effect. ACS Appl Mater Interfaces 12:47099–47107. https://doi.org/10.1021/acsami.0c15482
Yuan M, An J, Zhang G et al (2022) In-situ nitrogen-doped carbon dots for fluorescence sensing of tetracycline antibiotic. Ceram Int 48:4047–4054. https://doi.org/10.1016/j.ceramint.2021.10.194
**e H, Lu Y, You R et al (2022) Green synthetic nitrogen-doped graphene quantum dot fluorescent probe for the highly sensitive and selective detection of tetracycline in food samples. RSC Adv 12:8160–8171. https://doi.org/10.1039/d2ra00337f
Yan F, Sun Z, Pang J et al (2020) Functionalized carbon dots of thiazole derivatives based on inner filter effect for tetracyclines detection. Dyes Pigm 183:108673. https://doi.org/10.1016/j.dyepig.2020.108673
Zhao C, Jiao Y, Gao Z et al (2018) N, S co-doped carbon dots for temperature probe and the detection of tetracycline based on the inner filter effect. J Photochem Photobiol Chem 367:137–144. https://doi.org/10.1016/j.jphotochem.2018.08.023
Zhang H, Zhou Q, Han X et al (2021) Nitrogen-doped carbon dots derived from hawthorn for the rapid determination of chlortetracycline in pork samples. Spectrochim Acta Mol Biomol Spectrosc 255:119736. https://doi.org/10.1016/j.saa.2021.119736
Gu B, Chen D, Gao B et al (2020) Ultrasensitive Fluorescent Detection of Tetracycline Based on Selective Supramolecular Interaction of Nitrogen Chlorine Co–Doped Graphene Quantum dots. ChemistrySelect 5:7155–7163. https://doi.org/10.1002/slct.202000816
Zhao N, Wang Y, Hou S, Zhao L (2020) Functionalized carbon quantum dots as fluorescent nanoprobe for determination of tetracyclines and cell imaging. Microchim Acta 187:351. https://doi.org/10.1007/s00604-020-04328-1
Tang X, Wang L, Ye H et al (2022) Biological matrix-derived carbon quantum dots: highly selective detection of tetracyclines. J Photochem Photobiol Chem 424. https://doi.org/10.1016/j.jphotochem.2021.113653
Qian S, Qiao L, Xu W et al (2019) An inner filter effect-based near-infrared probe for the ultrasensitive detection of tetracyclines and quinolones. Talanta 194:598–603. https://doi.org/10.1016/j.talanta.2018.10.097
Gunjal DB, Gore AH, Bhosale AR et al (2019) Waste derived sustainable carbon nanodots as a new approach for sensitive quantification of ethionamide and cell imaging. J Photochem Photobiol Chem 376:54–62. https://doi.org/10.1016/j.jphotochem.2019.02.031
Ge J, Ma D, Duan G et al (2022) A novel probe for tetracyclines detection and its applications in cell imaging based on fluorescent WS2 quantum dots. Anal Chim Acta 1221:340130. https://doi.org/10.1016/j.aca.2022.340130
Fan Y, Qiao W, Long W et al (2022) Detection of tetracycline antibiotics using fluorescent turn-off sensor based on S, N-doped carbon quantum dots. Spectrochim Acta Mol Biomol Spectrosc 274:121033. https://doi.org/10.1016/j.saa.2022.121033
Daly B, Ling J, De Silva AP (2015) Current developments in fluorescent PET (photoinduced electron transfer) sensors and switches. Chem Soc Rev 44:4203–4211. https://doi.org/10.1039/c4cs00334a
Shi T, Tan L, Fu H, Wang J (2019) Application of molecular imprinting polymer anchored on CdTe quantum dots for the detection of sulfadiazine in seawater. Mar Pollut Bull 146:591–597. https://doi.org/10.1016/j.marpolbul.2019.07.010
Yu X, Meng Y, Yan Y et al (2020) Ethylenediamine functionalized MoS2 quantum dots for terramycin sensing in environmental water and fish samples. Microchem J 152. https://doi.org/10.1016/j.microc.2019.104406
Zhu Y, Lu Y, Shi L, Yang Y (2020) β-Cyclodextrin functionalized N,Zn codoped carbon dots for specific fluorescence detection of fluoroquinolones in milk samples. Microchem J 153. https://doi.org/10.1016/j.microc.2019.104517
Wang W, Deng P, Liu X et al (2021) A CsPbBr3 quantum dots/ultra-thin BN fluorescence sensor for stability and highly sensitive detection of tetracycline. Microchem J 162. https://doi.org/10.1016/j.microc.2020.105876
Sri S, Singh U, Kumar R et al (2021) Ignition of photoluminescent intensity of quenched MoS2 quantum dots tetracycline mixture by levofloxacin via photoinduced electron transfer. JCIS Open 3:100021. https://doi.org/10.1016/j.jciso.2021.100021
Tan X, Li Q, Yang J (2020) A simple fluorescence method detection levofloxacin in milk based on GSH-CdTe QDs. J Mol Struct 1201. https://doi.org/10.1016/j.molstruc.2019.127175
Chen XX, Lin ZZ, Yao QH, Huang ZY (2020) A practical aptaprobe for sulfadimethoxine residue detection in water and fish based on the fluorescence quenching of CdTe QDs by poly(diallyldimethylammonium chloride). J Food Compos Anal 91:103526. https://doi.org/10.1016/j.jfca.2020.103526
Elmizadeh H, Soleimani M, Faridbod F, Bardajee GR (2018) Fabrication and optimization of a sensitive tetracycline fluorescent nano-sensor based on oxidized starch polysaccharide biopolymer-capped CdTe/ZnS quantum dots: box–behnken design. J Photochem Photobiol Chem 367:188–199. https://doi.org/10.1016/j.jphotochem.2018.08.021
Hashmi SZH, Dhiman TK, Chaudhary N et al (2021) Levofloxacin detection using l-Cysteine capped MgS Quantum dots via the Photoinduced Electron transfer process. Front Nanatechnol 3. https://doi.org/10.3389/fnano.2021.616186
Chaudhary N, Verma D, Gopal Sharma J, Solanki PR (2022) A novel bioinspired carbon quantum dots based optical sensor for ciprofloxacin detection. Mater Lett 308:131090. https://doi.org/10.1016/j.matlet.2021.131090
Maillard J, Klehs K, Rumble C et al (2021) Universal quenching of common fluorescent probes by water and alcohols. Chem Sci 12:1352–1362. https://doi.org/10.1039/d0sc05431c
Wang S, Zhao Q, Hazarika A et al (2023) Thermal tolerance of perovskite quantum dots dependent on A-site cation and surface ligand. Nat Commun 14:2216. https://doi.org/10.1038/s41467-023-37943-6
Zhang T, Li W, Huang K et al (2021) Regulation of functional groups on graphene quantum dots directs selective CO2 to CH4 conversion. Nat Commun 12:5265. https://doi.org/10.1038/s41467-021-25640-1
Chullasat K, Nurerk P, Kanatharana P et al (2018) A facile optosensing protocol based on molecularly imprinted polymer coated on CdTe quantum dots for highly sensitive and selective Amoxicillin detection. Sens Actuators B Chem 254:255–263. https://doi.org/10.1016/j.snb.2017.07.062
Wei X, Xu G, Gong C et al (2018) Fabrication and evaluation of sulfanilamide-imprinted composite sensors by develo** a custom-tailored strategy. Sens Actuators B Chem 255:2697–2703. https://doi.org/10.1016/j.snb.2017.09.081
Malik R, Pinnaka AK, Kaur M et al (2019) Water-soluble glutathione-CdS QDs with exceptional antimicrobial properties synthesized via green route for fluorescence sensing of fluoroquinolones. J Chem Technol Biotechnol 94:1082–1090. https://doi.org/10.1002/jctb.5855
Hong Y, Lam JWY, Tang BZ (2011) Aggregation-induced emission. Chem Soc Rev 40:5361–5388
Tong H, Hong Y, Dong Y et al (2006) Fluorescent light-up bioprobes based on tetraphenylethylene derivatives with aggregation-induced emission characteristics. Chem Commun 3705–3707. https://doi.org/10.1039/b608425g
Ruff Y, Lehn JM (2008) Glycodynamers: fluorescent dynamic analogues of polysaccharides. Angewandte Chemie - Int Ed 47:3556–3559. https://doi.org/10.1002/anie.200703490
Li L, Shi L, Jia J et al (2021) Red fluorescent carbon dots for tetracycline antibiotics and pH discrimination from aggregation-induced emission mechanism. Sens Actuators B Chem 332. https://doi.org/10.1016/j.snb.2021.129513
Bigdeli A, Ghasemi F, Abbasi-Moayed S et al (2019) Ratiometric fluorescent nanoprobes for visual detection: design principles and recent advances - a review. Anal Chim Acta 1079:30–58
Li W, Zhu J, **e G et al (2018) Ratiometric system based on graphene quantum dots and Eu3 + for selective detection of tetracyclines. Anal Chim Acta 1022:131–137. https://doi.org/10.1016/j.aca.2018.03.018
Meng L, Lan C, Liu Z et al (2019) A novel ratiometric fluorescence probe for highly sensitive and specific detection of chlorotetracycline among tetracycline antibiotics. Anal Chim Acta 1089:144–151. https://doi.org/10.1016/j.aca.2019.08.065
Jalili R, Khataee A (2020) Application of molecularly imprinted polymers and dual-emission carbon dots hybrid for ratiometric determination of chloramphenicol in milk. Food Chem Toxicol 146. https://doi.org/10.1016/j.fct.2020.111806
Jia L, Chen R, Xu J et al (2021) A stick-like intelligent multicolor nano-sensor for the detection of tetracycline: the integration of nano-clay and carbon dots. J Hazard Mater 413. https://doi.org/10.1016/j.jhazmat.2021.125296
Zhuang Y, Lin B, Yu Y et al (2021) A ratiometric fluorescent probe based on sulfur quantum dots and calcium ion for sensitive and visual detection of doxycycline in food. Food Chem 356. https://doi.org/10.1016/j.foodchem.2021.129720
Zhu J, Chu H, Shen J et al (2021) Green preparation of carbon dots from plum as a ratiometric fluorescent probe for detection of doxorubicin. Opt Mater (Amst) 114. https://doi.org/10.1016/j.optmat.2021.110941
Farouk F, Azzazy HME, Niessen WMA (2015) Challenges in the determination of aminoglycoside antibiotics, a review. Anal Chim Acta 890:21–43. https://doi.org/10.1016/j.aca.2015.06.038
Jia P, Bu T, Sun X et al (2019) A sensitive and selective approach for detection of tetracyclines using fluorescent molybdenum disulfide nanoplates. Food Chem 297:124969. https://doi.org/10.1016/j.foodchem.2019.124969
Guo X, Liu Y, Dong W et al (2021) Azithromycin detection in cells and tablets by N,S co-doped carbon quantum dots. Spectrochim Acta Mol Biomol Spectrosc 252:119506. https://doi.org/10.1016/j.saa.2021.119506
Qi H, Teng M, Liu M, Liu S, Li J, Yu H, Teng C, Huang Z, Liu H, Shao Q, Umar A, Ding T, Gao Q and Guo Z (2019) Biomass-derived nitrogen-doped carbon quantum dots: highly selective fluorescent probe for detecting Fe3+ ions and tetracyclines. J Colloid Interface Sci 539:332–41. https://doi.org/10.1016/j.jcis.2018.12.047
Feng Y, Zhong D, Miao H, Yang X (2015) Carbon dots derived from rose flowers for tetracycline sensing. Talanta 140:128–33. https://doi.org/10.1016/j.talanta.2015.03.038
Gunjal DB, Gurav YM, Gore AH, Naik VM, Waghmare RD, Patil CS, Sohn D, Anbhule PV, Shejwal RV, Kolekar GB (2019) Nitrogen doped waste tea residue derived carbon dots for selective quantification of tetracycline in urine and pharmaceutical samples and yeast cell imaging application. Opt Mater (Amst) 98:109484. https://doi.org/10.1016/j.optmat.2019.109484
Anderson VE, Osheroff N (2001) Type II topoisomerases as targets for Quinolone antibacterials: turning Dr. Jekyll into Mr. Hyde. Curr Pharm Des 7:339–355
Hooper DC (2001) Emerging mechanisms of Fluoroquinolone Resistance. Emerg Infect Disease 7:337–341
Fu Y, Zhao S, Wu S et al (2020) A carbon dots-based fluorescent probe for turn-on sensing of ampicillin. Dyes Pigm 172:107846. https://doi.org/10.1016/j.dyepig.2019.107846
Fu Y, Zhao S, Wu S, Huang L, Xu T, **ng X, Lan M, Song X (2020) A carbon dots-based fluorescent probe for turn-on sensing of ampicillin Dyes and Pigments 172:107846. https://doi.org/10.1016/j.dyepig.2019.107846
Guo X, Zhang L, Wang Z, Sun Y, Liu Q, Dong W, Hao A (2019) Fluorescent carbon dots based sensing system for detection of enrofloxacin in water solutions. Spectrochim Acta A Mol Biomol Spectrosc 219:15–22. https://doi.org/10.1016/j.saa.2019.02.017
Dang VD, Ganganboina AB, Doong RA (2020) Bipyridine- and copper-functionalized n-doped carbon dots for fluorescence turn off-on detection of ciprofloxacin ACS Appl Mater Interfaces 12:32247–58. https://doi.org/10.1021/acsami.0c04645
Aldred KJ, McPherson SA, Turnbough CL et al (2013) Topoisomerase IV-quinolone interactions are mediated through a water-metal ion bridge: mechanistic basis of quinolone resistance. Nucleic Acids Res 41:4628–4639. https://doi.org/10.1093/nar/gkt124
Wang K, Ji Q, Xu J, Li H, Zhang D, Liu X, Wu Y, Fan H (2018) Highly sensitive and selective detection of amoxicillin using carbon quantum dots derived from beet. J Fluoresc 28:759–765. https://doi.org/10.1007/s10895-018-2237-0
Zhang L, Wang J, Deng J and Wang S (2020) A novel fluorescent “turn-on” aptasensor based on nitrogen-doped graphene quantum dots and hexagonal cobalt oxyhydroxide nanoflakes to detect tetracycline. Anal Bioanal Chem 412:1343–1351. https://doi.org/10.1007/s00216-019-02361-5
Venkatalaxmi A, Padmavathi BS, Amaranath T (2004) A general solution of unsteady Stokes equations. Fluid Dyn Res 35:229–236. https://doi.org/10.1016/j.uiddyn.2004.06.001
Wang S, Wang W, Li M et al (2022) On-site, rapid, and facile determination of gentamicin using a fluorescent resonance energy transfer sensor constructed from nitrogen-carbon quantum dots functionalized by 4,5-imidazole dicarboxylic acid. Food Chem 371:131366. https://doi.org/10.1016/j.foodchem.2021.131366
Filist M, Buś-Kwaśnik K, Ksycińska H, Rudzki PJ (2014) Simplified LC-MS/MS method enabling the determination of azithromycin in human plasma after a low 100 mg dose administration. J Pharm Biomed Anal 100:184–189. https://doi.org/10.1016/j.jpba.2014.07.015
Waetzig V, Riffert J, Cordt J et al (2017) Neurodegenerative effects of azithromycin in differentiated PC12 cells. Eur J Pharmacol 809:1–12. https://doi.org/10.1016/j.ejphar.2017.05.002
Ebrahimzadeh H, Yamini Y, Ara KM et al (2010) Determination of azithromycin in biological samples by LLLME combined with LC. Chromatographia 72:731–735. https://doi.org/10.1365/s10337-010-1692-9
Gandhi R, Kaul CL, Panchagnula R (2000) Validated LC method for in-vitro analysis of azithromycin using electrochemical detection. J Pharm Biomed Anal 23:1073–1079
Kennedy WPU, Wallace AT, Murdoch JMC (1963) Ampicillin in treatment of certain gram-negative bacterial infections. Br Med J 2:962–965
Klotz U, Schwab M (2005) Topical delivery of therapeutic agents in the treatment of inflammatory bowel disease. Adv Drug Deliv Rev 57:267–279
Sudewi S, Chabib L, Zulfajri M, Gedda G, Huang GG (2023) Polyvinylpyrrolidone-passivated fluorescent iron oxide quantum dots for turn-off detection of tetracycline in biological fluids. J Food Drug Anal 31:177–93. https://doi.org/10.38212/2224-6614.3440
Sudewi S, Zulfajri M, Dayalan S, Hsu SCN, Huang GG (2023) Glutamic acid–capped iron oxide quantum dots as fluorescent nanoprobe for tetracycline in urine Microchimica Acta. 190:226. https://doi.org/10.1007/s00604-023-05801-3
Ju J, Chen W (2014) Synthesis of highly fluorescent nitrogen-doped graphene quantum dots for sensitive, label-free detection of Fe (III) in aqueous media. Biosens Bioelectron 58:219–225. https://doi.org/10.1016/j.bios.2014.02.061
Yang X, Luo Y, Zhu S et al (2014) One-pot synthesis of high fluorescent carbon nanoparticles and their applications as probes for detection of tetracyclines. Biosens Bioelectron 56:6–11. https://doi.org/10.1016/j.bios.2013.12.064
Yan Y, Liu JH, Li RS et al (2019) Carbon dots synthesized at room temperature for detection of tetracycline hydrochloride. Anal Chim Acta 1063:144–151. https://doi.org/10.1016/j.aca.2019.02.047
Hooper DC, Jacoby GA (2015) Mechanisms of drug resistance: quinolone resistance. Ann N Y Acad Sci 1354:12–31. https://doi.org/10.1111/nyas.12830
Lin M, Zou HY, Yang T et al (2016) An inner filter effect based sensor of tetracycline hydrochloride as developed by loading photoluminescent carbon nanodots in the electrospun nanofibers. Nanoscale 8:2999–3007. https://doi.org/10.1039/c5nr08177g
Harris ED (1990) Rheumatoid arthritis: pathophysiology and implications for Therapy. N Engl J Med 322:1277–1289
Funding
The study was supported by the Taiwan National Science and Technology Council under grants NSTC112-2113-M-037-0071. This study was also funded by a grant from the Kaohsiung Medical University Research Foundation (KMU-M113013).
Author information
Authors and Affiliations
Contributions
All authors contributed to the review idea and design. Conceptualization, methodology, investigation, formal analysis, and writing-original draft preparation were performed by Sri Sudewi. Data curation and project administration was handled by Penki Venkata Sai Sashank. Software and visualization of data were provided by Rajiv Kamaraj. Validation, methodology, and writing-reviewing and editing section were performed by Muhammad Zulfajri. Supervision, resources, funding acquisition, and writing-reviewing and editing were responded by Genin Gary Huang. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sudewi, S., Sai Sashank, P.V., Kamaraj, R. et al. Understanding Antibiotic Detection with Fluorescence Quantum Dots: A Review. J Fluoresc (2024). https://doi.org/10.1007/s10895-024-03743-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10895-024-03743-4