Abstract
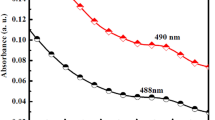
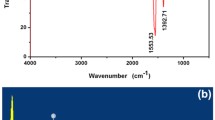
The CdS quantum dots (QDs) were prepared by rapid, one-pot, and novel photochemical method, which used Thioglycolic acid (TGA) molecules as both stabilizer and sulfur source. The structure and morphology of the prepared CdS QDs were characterized by different analyses such as XRD, FT-IR, Raman, EDS, TEM, PL, and absorption. In this work, was used of CdS QDs as off fluorescence sensor for rapid and simple detection of lead (Pb2+) ions in water. The PL intensity of CdS QDs in the presence of lead ions decreased gradually and in the presence of 100 μM lead ions, photo emission completely quenched. The photocatalyst performance of CdS QDs was investigated by methylene blue (MB), methylene orange (MO), and rhodamine b (RB) pollutant dyes under both UV and sun lights. The obtained results showed that CdS QDs had excellent photocatalyst activity with dyes under UV light and 94.9% of MO dye, 94.4% of RB dye, and 81.2% of MB was degraded after 60 min UV irradiation. For understanding about which parameter have a key role in the photodegradation process of MO by CdS QDs under UV illumination, several radical scavengers were used, and results showed that holes have a key role in the degradation process.











Similar content being viewed by others
Availability of Data and Material/ Data Availability
All data of this paper are available and included in the manuscript. This work was done in the nanoscience lab of the Vali-e-Asr University of Rafsanjan, Iran, this article is original, this article has been written by the stated authors who are ALL aware of its content and approve its submission. This article has not been published previously.
References
Farahmandzadeh F, Molaei M, Alehdaghi H, Karimipour M (2022) The significant increasing photoluminescence quantum yield of the CdTe/CdS/ZnS core/multi-shell quantum dots (QDs) by 60Co gamma irradiation. Appl Phys A 128(3):1–10
Samadpour M, Irajizad A, Taghavinia N, Molaei M (2011) A new structure to increase the photostability of CdTe quantum dot sensitized solar cells. J Phys D Appl Phys 44(4):045103
He Y, Hao-Ting Lu, Sai L-M, Yuan-Yuan Su, Mei Hu, Fan C-H, Huang W, Wang L-H (2008) Microwave synthesis of water-dispersed CdTe/CdS/ZnS core-shell-shell quantum dots with excellent photostability and biocompatibility. Adv Mater 20(18):3416–3421
Qiu Z, Jian Shu Yu, He ZL, Zhang K, Lv S, Tang D (2017) CdTe/CdSe quantum dot-based fluorescent aptasensor with hemin/G-quadruplex DNzyme for sensitive detection of lysozyme using rolling circle amplification and strand hybridization. Biosens Bioelectron 87:18–24
Parand P, Rezagholipour DH (2014) Tuning the luminescence of CdS quantum dots by a simple method. J Nanostruc 4:193–197
Marandi M, Taghavinia N, Mahdavi SM (2008) Self-assembled one-pot synthesis of red luminescent CdS: Mn/Mn (OH) 2 nanoparticles. J Lumin 128(12):1980–1984
Abdulla MM, Hasan NH, Mohammed HI, Mohamed GH, Al-Hamdani KA, Abdulameer AF (2012) Investigation of optical properties of the PbS/CdS thin films by thermal evaporation. J Electron Devices 12:761–766
Chang CM, Orchard KL, Martindale BC, Reisner E (2016) Ligand removal from CdS quantum dots for enhanced photocatalytic H 2 generation in pH neutral water. J Mater Chem A 4(8):2856–2862
Bansal AK, Antolini F, Zhang S, Stroea L, Ortolani L, Lanzi M, Serra E, Allard S, Scherf U, Samuel ID (2016) Highly luminescent colloidal CdS quantum dots with efficient near-infrared electroluminescence in light-emitting diodes. J Phys Chem C 120(3):1871–1880
Vossmeyer T, Katsikas L, Giersig M, Popovic IG, Diesner K, Chemseddine A, Eychmüller A, Weller H (1994) CdS nanoclusters: synthesis, characterization, size dependent oscillator strength, temperature shift of the excitonic transition energy, and reversible absorbance shift. J Phys Chem 98(31):7665–7673
Wang Z, **ng X, Yang Y, Zhao R, Zou T, Wang Z, Wang Y (2018) One-step hydrothermal synthesis of thioglycolic acid capped CdS quantum dots as fluorescence determination of cobalt ion. Sci Rep 8(1):1–12
Zhou C, Zhou L, Jiehua Xu, Gan Y (2016) Controllable synthesis of CdS quantum dots and their photovoltaic application on quantum-dot-sensitized ZnO nanorods. J Solid State Electrochem 20(2):533–540
Horoz S, Sahin O (2017) Synthesis, characterizations and photovoltaic properties of Cr-doped CdS QDs. J Mater Sci Mater Electron 28(23):17784–17790
Sharma P, Dubey RS (2005) Lead toxicity in plants. Braz J Plant Physiol 17:35–52
Carocci A, Catalano A, Lauria G, Sinicropi MS, Genchi G (2016) Lead toxicity, antioxidant defense and environment. Rev Environ Contam Toxicol 238:45–67. https://doi.org/10.1007/398_2015_5003
Faisal AA, Al-Wakel SF, Assi HA, Naji LA, Naushad M (2020) Waterworks sludge-filter sand permeable reactive barrier for removal of toxic lead ions from contaminated groundwater. J Water Process Eng 33:101112
Boruah BS, Biswas R (2018) An optical fiber based surface plasmon resonance technique for sensing of lead ions: A toxic water pollutant. Opt Fiber Technol 46:152–156
Meng A, Qinhai Xu, Zhao K, Li Z, Liang J, Li Q (2018) A highly selective and sensitive “on-off-on” fluorescent probe for detecting Hg (II) based on Au/N-doped carbon quantum dots. Sens Actuators B Chem 255:657–665
Paisuwan W, Lertpiriyasakulkit T, Ruangpornvisuti V, Sukwattanasinitt M, Ajavakom A (2020) 8-Hydroxyjulolidine aldimine as a fluorescent sensor for the dual detection of Al3+ and Mg2+. Sens Bio-Sens Res 29:100358
Zou T, **ng X, Yang Y, Wang Z, Wang Z, Zhao R, Zhang X, Wang Y (2020) Water-soluble ZnO quantum dots modified by (3-aminopropyl) triethoxysilane: The promising fluorescent probe for the selective detection of Cu2+ ion in drinking water. J Alloy Compd 825:153904
Yadav N, Kumar R, Singh AK, Mohiyuddin S, Gopinath P (2020) Systematic approach of chromone skeleton for detecting Mg2+, ion: Applications for sustainable cytotoxicity and cell imaging possibilities. Spectrochim Acta Part A Mol Biomol Spectrosc 235:118290
Liu Z, ** W, Wang F, Li T, Nie J, **ao W, Zhang Q, Zhang Y (2019) Ratiometric fluorescent sensing of Pb2+ and Hg2+ with two types of carbon dot nanohybrids synthesized from the same biomass. Sens Actuators B Chem 296:126698
Zhang Q, Ma R, Li Z, Liu Z (2020) A multi-responsive crown ether-based colorimetric/fluorescent chemosensor for highly selective detection of Al3+, Cu2+ and Mg2+. Spectrochim Acta Part A Mol Biomol Spectrosc 228:117857
He K, **aoxiao Yu, Qin L, Yiwei Wu (2022) CdS QDs: Facile synthesis, design and application as an “on–off” sensor for sensitive and selective monitoring Cu2+, Hg2+ and Mg2+ in foods. Food Chem 390:133116
Qi P, Zhang D, Zeng Y, Wan Yi (2016) Biosynthesis of CdS nanoparticles: A fluorescent sensor for sulfate-reducing bacteria detection. Talanta 147:142–146
Tedsana W, Tuntulani T, Ngeontae W (2013) A highly selective turn-on ATP fluorescence sensor based on unmodified cysteamine capped CdS quantum dots. Anal Chim Acta 783:65–73
Farahmandzadeh F, Molaei M, Karimipour M (2022) Ultrafast synthesis of CdTe/ZnSe semiconductor QDs by microwave method and investigation of structural, optical, and photocatalytic properties of CdTe/ZnSe QDs. J Mater Sci Mater Electron 33:95–104. https://doi.org/10.1007/s10854-021-07255-w
Li D, Wang S, Wang J, Zhang X, Liu S (2013) Synthesis of CdTe/TiO2 nanoparticles and their photocatalytic activity. Mater Res Bull 48(10):4283–4286
Molaei M, Farahmandzadeh F, Mousavi TS, Karimipour M (2021) Photochemical synthesis, investigation of optical properties and photocatalytic activity of CdTe/CdSe core/shell quantum dots. Mater Technol 1818–1824. https://doi.org/10.1080/10667857.2021.1988040
Gupta VK, Saravanan R, Agarwal S, Gracia F, Khan MM, Qin J, Mangalaraja RV (2017) Degradation of azo dyes under different wavelengths of UV light with chitosan-SnO2 nanocomposites. J Mol Liq 232:423–430
Farahmandzadeh F, Molaei M (2022) CdSe/CdS/ZnS core/multi-shell QDs: new microwave synthesis and applications for dye photodegradations. J Coord Chem 524–534. https://doi.org/10.1080/00958972.2022.2056698
Hussain W, Malik H, Bahadur A, Hussain RA, Shoaib M, Iqbal S, Hussain H, Green IR, Badshah A, Li H (2018) Synthesis and characterization of CdS photocatalyst with different morphologies: visible light activated dyes degradation study. Kinet Catal 59(6):710–719
Li **, Zhu J, Li H (2012) Comparative study on the mechanism in photocatalytic degradation of different-type organic dyes on SnS2 and CdS. Appl Catal B 123:174–181
Liu Li, Yue M, **rong Lu, **shan Hu, Liang Y, Cui W (2018) The enrichment of photo-catalysis via self-assembly perylenetetracarboxylic acid diimide polymer nanostructures incorporating TiO2 nano-particles. Appl Surf Sci 456:645–656
Zyoud A, Zu’bi A, Helai MH, Park D, Campet G, Hilal HS (2015) Optimizing photo-mineralization of aqueous methyl orange by nano-ZnO catalyst under simulated natural conditions. J Environ Health Sci Eng 13(1):1–10
Mahvelati-Shamsabadi T, Goharshadi EK, Shafaee M, Niazi Z (2018) ZnS@ reduced graphene oxide nanocomposite as an effective sunlight driven photocatalyst for degradation of reactive black 5: a mechanistic approach. Sep Purif Technol 202:326–334
Talukdar S, Dutta RK (2016) A mechanistic approach for superoxide radicals and singlet oxygen mediated enhanced photocatalytic dye degradation by selenium doped ZnS nanoparticles. RSC Adv 6(2):928–936
Karimipour M, Kheshabnia A, Molaei M (2016) Red luminescence of Zn/ZnO core–shell nanorods in a mixture of LTZA/Zinc acetate matrix: Study of the effects of Nitrogen bubbling, Cobalt do** and thioglycolic acid. J Lumin 178:234–240
Gong K, Kelley DF, Kelley AM (2017) Resonance Raman excitation profiles of CdS in pure CdS and CdSe/CdS core/shell quantum dots: CdS-localized excitons. J Chem Phys 147(22):224702
Milekhin AG, Sveshnikova LL, Duda TA, Surovtsev NV, Adichtchev SV, Zahn DR (2008) Surface enhanced Raman scattering by CdS quantum dots. JETP Lett 88(12):799–801
Ben Brahim N, Poggi M, Lambry JC, Bel Haj Mohamed N, Ben Chaâbane R, Negrerie M (2018) Density of grafted chains in thioglycerol-capped CdS quantum dots determines their interaction with aluminum (III) in water. Inorg Chem 57(9):4979–4988
Khairy M, Ayoub HA, Banks CE (2018) Large-scale production of CdO/Cd (OH) 2 nanocomposites for non-enzyme sensing and supercapacitor applications. RSC Adv 8(2):921–930
Wang K, Liu Q, Dai L, Yan J, Chang Ju, Qiu B, **angyang Wu (2011) A highly sensitive and rapid organophosphate biosensor based on enhancement of CdS–decorated graphene nanocomposite. Anal Chim Acta 695(1–2):84–88
Du P, Li H, Mei Z, Liu S (2009) Electrochemical DNA biosensor for the detection of DNA hybridization with the amplification of Au nanoparticles and CdS nanoparticles. Bioelectrochemistry 75(1):37–43
An X, Yu X, Jimmy CY, Zhang G (2013) CdS nanorods/reduced graphene oxide nanocomposites for photocatalysis and electrochemical sensing. J Mater Chem A 1(16):5158–5164
Han Z, Wang M, Chen X, Shen S (2016) CdSe-sensitized branched CdS hierarchical nanostructures for efficient photoelectrochemical solar hydrogen generation. Phys Chem Chem Phys 18(16):11460–11466
Rathinamala I, Babu IM, William JJ, Muralidharan G, Prithivikumaran N (2020) CdS microspheres as promising electrode materials for high performance supercapacitors. Mater Sci Semicond Process 105:104677
Yu J, Song Na, Zhang Y-K, Zhong S-X, Wang A-J, Chen J (2015) Green preparation of carbon dots by **hua bergamot for sensitive and selective fluorescent detection of Hg2+ and Fe3+. Sens Actuators B Chem 214:29–35
Li S, Li Y, Cao J, Zhu J, Fan L, Li X (2014) Sulfur-doped graphene quantum dots as a novel fluorescent probe for highly selective and sensitive detection of Fe3+. Anal Chem 86(20):10201–10207
Mahapatra N, Panja S, Mandal A, Halder M (2014) A single source-precursor route for the one-pot synthesis of highly luminescent CdS quantum dots as ultra-sensitive and selective photoluminescence sensor for Co 2+ and Ni 2+ ions. J Mater Chem C 2(35):7373–7384
Nguyen DC, Tien K-Y, Won-Chun Oh (2017) Synthesis of frost-like CuO combined graphene-TiO2 by self-assembly method and its high photocatalytic performance. Appl Surf Sci 412:252–261
Nguyen DC, Tien K-Y, Won-Chun Oh (2022) Synthesis of frost-like CuO combined graphene-TiO2 by self-assembly method and its high photocatalytic performance. Appl Surf Sci 412:252–261
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Farzad Farahmandzadeh, Samira Salehi, Mehdi Molaei, Haniyeh Fallah, and Vajihe Nejadshafiee. The first draft of the manuscript was written by Farzad Farahmandzadeh. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent to Participate
This article has been written by the stated authors who are ALL aware of its content and approve its submission.
Consent to Publication
This study doesn’t contain any data from an individual person.
Competing Interests
The authors declare they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Farahmandzadeh, F., Salehi, S., Molaei, M. et al. CdS Semiconductor Quantum Dots; Facile Synthesis, Application as Off Fluorescent Sensor for Detection of Lead (Pb2+) Ions and Catalyst for Degradation of Dyes from Water. J Fluoresc 33, 1515–1524 (2023). https://doi.org/10.1007/s10895-023-03157-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-023-03157-8