Abstract
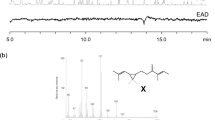
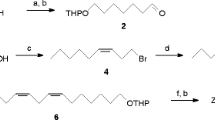
The cotton mealybug, Phenacoccus solenopsis, the distribution of which was formerly limited to Nearctic and Neotropical regions, recently invaded many countries in various regions including Asia, Africa, and the Pacific. More recently, P. solenopsis was newly recorded in Japan and is currently an emerging pest of agricultural crops. In this study, we determined the structure of a sex pheromone of P. solenopsis in order to develop an effective lure for monitoring this pest. From volatiles emitted by virgin adult females, we isolated a compound attractive to males. By means of coupled gas chromatography–mass spectrometry and nuclear magnetic resonance spectroscopy, we identified this as (2,2-dimethyl-3-isopropylidenecyclobutyl)methyl 3-methylbut-2-enoate. This compound was synthesized and shown to be attractive to male P. solenopsis. Analysis by gas chromatography using an enantioselective stationary phase and polarimetry analyses of the natural pheromone and synthetic enantiomers showed the natural compound to be the (R)-(−)-enantiomer. This compound is an ester of maconelliol, which has an unusual cyclobutane structure found in sex pheromones of other mealybug species, and senecioic acid, also found in the pheromones of other mealybug species. However, this is the first example of the ester of maconelliol and senecioic acid as a natural product.





Similar content being viewed by others
References
Abbas G, Arif MJ, Saeed S (2005) Systematic status of a new species of the genus Phenacoccus Cockerell (Pseudococcidae), a serious pest of cotton, Gossypium hirsutum L., in Pakistan. Pak Entomol 27:83–84
Arai T (2000) The existence of sex pheromone of Pseudococcus cryptus Hempel (Homoptera: Pseudococcidae) and a simple bioassay. Appl Entomol Zool 35:525–528
Arai T, Sugie H, Hiradate S, Kuwahara S, Itagaki N, Nakahata T (2003) Identification of a sex pheromone component of Pseudococcus cryptus. J Chem Ecol 29:2213–2223
Ben-Dov Y (1994) A systematic catalogue of the mealybugs of the world. Intercept, Andover, 686 p
Bierl-Leonhard BA, Moreno DS, Schwarz M, Fargerlund J, Plimmer JR (1981) Isolation, identification, and synthesis of the sex pheromone of the citrus mealybug, Planococcus citri (Risso). Tetrahedron Lett 22:389–392
Breitmaier E (2006) Terpenes. Wiley-VCH, Weinheim, 223 p
Da Porto C, Decorti D, Kikic I (2009) Flavour compounds of Lavandula angustifolia L. To use in food manufacturing: comparison of three different extraction methods. Food Chem 112:1072–1078
García Morales M, Denno BD, Miller DR, Miller GL, Ben-Dov Y, Hardy NB (2016) ScaleNet: A literature-based model of scale insect biology and systematics. http://scalenet.info/ Accessed 27 July 2016
Gullan PJ, Downie DA, Steffan SA (2003) A new pest species of the mealybug genus Ferrisia Fullaway (Hemiptera: Pseudococcidae) from the United States. Ann Entomol Soc Am 96:723–737
Gunawardena K, Rivera SB, Epstein WW (2002) The monoterpenes of Artemisia tridentata ssp. vaseyana, Artemisia cana ssp. viscidula and Artemisia tridentata ssp. spiciformis. Phytochemistry 59:197–203
Gut LJ, Stelinski LL, Thomson DR, Miller JR (2004) Behaviour-modifying chemicals: prospects and constraints in IPM. In: Koul O, Dhaliwal GS, Cuperus GW (eds) Integrated pest management: potential, constraints and challenges. CABI Publishing, Oxford, pp. 73–122
Gutierrez AP, Ponti L, d’Oultremont T, Ellis CK (2008) Climate change effects on poikilotherm tritrophic interactions. Clim Chang 87:167–192
Hinkens DM, McElfresh JS, Millar JG (2001) Identification and synthesis of the sex pheromone of the vine mealybug, Planococcus ficus. Tetrahedron Lett 42:1619–1621
Hodgson C, Abbas G, Arif MJ, Saeed S, Karar H (2008) Phenacoccus solenopsis Tinsley (Sternorrhyncha: Coccoidea: Pseudococcidae), an invasive mealybug damaging cotton in Pakistan and India, with a discussion on seasonal morphological variation. Zootaxa 1913:1–35
Inada M (2016) Phenacoccus solenopsis Tinsley. Plant Protection Station, Ministry of Agriculture, Forestry and Fisheries Japan http://www.maff.go.jp/pps/j/guidance/pestinfo/pdf/pestinfo_108_6.pdf Accessed 27 July 2016
Jara V, Meza FJ, Zaviezo T, Chorbadjian R (2013) Climate change impacts on invasive potential of pink hibiscus mealybug, Maconellicoccus hirsutus (green), in Chile. Clim Chang 117:305–317
Logani MK, Varshney IP, Pandey RC, Dey S (1967) β-Cyclolavandulal, a new naturally occurring monoterpene type. Tetrahedron Lett 8:2645–2648
Millar JG, Daane KM, McElfresh JS, Moreira JA, Bentley WJ (2005) Chemistry and applications of mealybug sex pheromones. In: Petroski RJ, Tellez MR, Behle RW (eds) Semiochemicals in pest and weed control. American Chemical Society, Washington DC, pp. 11–27
R Development Core Team (2016) R: A language and environment for statistical computing. http://www.R-project.org/ Accessed on 15 April 2016
Sugie H, Teshiba M, Narai Y, Tsutsumi T, Sawamura N, Tabata J, Hiradate S (2008) Identification of a sex pheromone component of the Japanese mealybug, Planococcus kraunhiae (Kuwana). Appl Entomol Zool 43:369–375
Tabata J, Ichiki RT (2015) A new lavandulol-related monoterpene in the sex pheromone of the grey pineapple mealybug, Dysmicoccus neobrevipes. J Chem Ecol 41:194–201
Tabata J, Ichiki RT, Tanaka H, Kageyama D (2016) Sexual versus asexual reproduction: distinct outcomes in relative abundance of parthenogenetic mealybugs following recent colonization. Plos One 11:e0156587
Tabata J, Narai Y, Sawamura N, Hiradate S, Sugie H (2012) A new class of mealybug pheromones: a hemiterpene ester in the sex pheromone of Crisicoccus matsumotoi. Naturwissenschaften 99:567–574
Tabata J, Teshiba M, Shimizu N, Sugie H (2015) Mealybug mating disruption by a sex pheromone derived from lavender essential oil. J Essent Oil Res 27:232–237
Tanaka H, Tabata J (2014) A new record of Phenacoccus solenopsis Tinsley, 1898 from Kyushu district, Japan. Jpn J Entomol Soc 17:119–120
Tanaka H, Uesato T (2012) New records of some potential pest mealybugs (Hemiptera: Coccoidea: Pseudococcidae) in Japan. Appl Entomol Zool 47:413–419
Williams DJ (1996) A brief account of the hibiscus mealybug Maconellicoccus hirsutus (Hemiptera: Pseudococcidae), a pest of agriculture and horticulture, with descriptions of two related species from southern Asia. Bull Entomol Res 86:617–628
Zhang A, Nie J (2005) Enantioselective synthesis of the female sex pheromone of the pink hibiscus mealybug, Maconellicoccus hirsutus. J Agric Food Chem 53:2451–2455
Zhang A, Amalin D, Shirali S, Serrano MS, Franqui RA, Oliver JE, Klun JA, Aldrich JR, Meyerdirk DE, Lapointe SL (2004a) Sex pheromone of the pink hibiscus mealybug, Maconellicoccus hirsutus, contains an unusual cyclobutanoid monoterpene. Proc Natl Acad Sci U S A 101:9601–9606
Zhang A, Nie J, Khrimian A (2004b) Chiral synthesis of maconelliol: a novel cyclobutanoid terpene alcohol from pink hibiscus mealybug, Maconellicoccus hirsutus. Tetrahedron Lett 45:9401–9403
Zou Y, Millar JG (2015) Chemistry of the pheromones of scale and mealybug insects. Nat Prod Rep 32:1067–1113
Acknowledgments
We thank Dr. H. Tanaka (Kyushu University) for providing useful information regarding mealybug collection and taxonomy. NMR spectroscopy analyses were carried out with the support of Dr. S. Hiradate of the Advanced Analysis Center at NARO. J.T. gratefully acknowledges a grant-in-aid for scientific research from the Japan Society for the Promotion of Science (no. 16 K08103).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tabata, J., Ichiki, R.T. Sex Pheromone of the Cotton Mealybug, Phenacoccus solenopsis, with an Unusual Cyclobutane Structure. J Chem Ecol 42, 1193–1200 (2016). https://doi.org/10.1007/s10886-016-0783-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-016-0783-y