Abstract
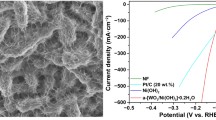
Although Urea oxidation reaction (UOR) with favorable thermodynamic potential has been well-acknowledged as a feasible alternative to slow oxygen evolution reaction for hydrogen production through water splitting, but the shortage of low-cost and high efficiency electrocatalysts restricts its practical development. In this research, a free-standing nickel–iron-phosphide (NiFeP) nanosheets electrocatalysts on nickel foam is synthesized by electrodeposition method. The presence of phosphide (P) causes nickel–iron (NiFe) to evolve from crystalline to amorphous structures. The NiFeP electrocatalysts with nanosheets structure exhibit reliable electrocatalytic activities toward UOR with small potential of 1.397 V at 10 mA cm−2, low Tafel slope of 60.1 mV dec−1 and outstanding catalytic stability in 1.0 M KOH with 0.33 M urea solution. More impressively, the electrochemical activation energy (Ea) of UOR for the NiFeP electrocatalyst (35.6 kJ mol−1) is much lower than that of NiFe electrocatalyst (52.1 kJ mol−1). The phosphorus do** triggers the electronic structure of Ni and Fe site reconstruction, which is conducive to accelerating charge and mass transport, and then contributes to the enhanced electrocatalytic performance. Furthermore, the formation of amorphous structure and nanosheets structure by the incorporation of phosphorus contributes to the improved intrinsic activity of NiFeP for UOR.







Similar content being viewed by others
Data availability
The data that support all plots within this paper are available from the corresponding author upon reasonable request.
References
Wu Y, Sun Z, Wang Y, Yin L, He Z, Zhang Z, Hayat MD, Zang Q, Lian J (2023) Cyclic voltammetric deposition of binder-free Ni-Se film on Ni foams as efficient bifunctional electrocatalyst for boosting overall urea-water electrolysis. J Alloys Compd 937:168460. https://doi.org/10.1016/j.jallcom.2022.168460
Sun F, Qin J, Wang Z, Yu M, Wu X, Sun X, Qiu J (2021) Energy-saving hydrogen production by chlorine-free hybrid seawater splitting coupling hydrazine degradation. Nat Commun 12:4182. https://doi.org/10.1038/s41467-021-24529-3
You S, Wu Y, Wang Y, He Z, Yin L, Zhang Y, Sun Z, Zhang Z (2022) Pulse-electrodeposited Ni–Fe–Sn films supported on Ni foam as an excellent bifunctional electrocatalyst for overall water splitting. Int J Hydrogen Energy 47:29315–29326. https://doi.org/10.1016/j.ijhydene.2022.06.265
Gujjula SR, Karingula S, Shajahan S, Siliveri S, Goskula S, Chirra S, Gobi KV, Narayanan V (2023) Rational design of metal organic framework derived porous Au@Co3O4/C nanocomposite materials for the electrochemical overall water splitting. J Mater Sci 58:9130–9147. https://doi.org/10.1007/s10853-023-08619-9
Ding Y, Du X, Zhang X (2022) Rose-like Cu-doped Ni3S2 nanoflowers decorated with thin NiFe LDH nanosheets for high-efficiency overall water and urea electrolysis. Appl Surf Sci 584:152622. https://doi.org/10.1016/j.apsusc.2022.152622
Zhang J, He T, Wang M, Qi R, Yan Y, Dong Z, Liu H, Wang H, **a BY (2019) Energy-saving hydrogen production coupling urea oxidation over a bifunctional nickel–molybdenum nanotube array. Nano Energy 60:894–902. https://doi.org/10.1016/j.nanoen.2019.04.035
Zhang Q, Sun M, Yao M, Zhu J, Yang S, Chen L, Sun B, Zhang J, Hu W, Zhao P (2022) Interfacial engineering of an FeOOH@Co3O4 heterojunction for efficient overall water splitting and electrocatalytic urea oxidation. J Colloid Interface Sci 623:617–626. https://doi.org/10.1016/j.jcis.2022.05.070
Jiang L, Pan Y, Zhang J, Chen X, Ye X, Li Z, Li C, Sun Q (2022) Mo propellant boosting the activity of Ni–P for efficient urea-assisted water electrolysis of hydrogen evolution. J Colloid Interface Sci 622:192–201. https://doi.org/10.1016/j.jcis.2022.04.050
Luong QT, Choi HJ, Huynh TBN, Song J, Cho Y, Kwon OJ (2022) Unraveling the formation of optimum point in NiCo-based electrocatalysts for urea oxidation reaction. Electrochim Acta 431:141159. https://doi.org/10.1016/j.electacta.2022.141159
Diao Y, Liu Y, Hu G, Zhao Y, Qian Y, Wang H, Shi Y, Li Z (2022) NiFe nanosheets as urea oxidation reaction electrocatalysts for urea removal and energy-saving hydrogen production. Biosens Bioelectron 211:114380. https://doi.org/10.1016/j.bios.2022.114380
Hu L, ** L, Zhang T, Zhang J, He J, Chen D, Li N, Xu Q, Lu J (2022) Self-supported MoO2–MoO3/Ni2P hybrids as a bifunctional electrocatalyst for energy-saving hydrogen generation via urea–water electrolysis. J Colloid Interface Sci 614:337–344. https://doi.org/10.1016/j.jcis.2022.01.129
Samdani JS, Sanetuntikul J, Shanmugam S (2022) Self-supported iron-doped nickel sulfide as efficient catalyst for electrochemical urea and hydrazine oxidation reactions. Int J Hydrogen Energy 47:27347–27357. https://doi.org/10.1016/j.ijhydene.2022.06.073
Kang J, Sheng C, Wang J, Xu H, Zhao B, Chen S, Qing Y, Wu Y (2023) Natural-wood-fiber-assisted synthesis of Ni2P embedded in P-doped porous carbon as highly active catalyst for urea electro-oxidation. Int J Hydrogen Energy 48:7644–7654. https://doi.org/10.1016/j.ijhydene.2022.11.210
Zhang X, Fang X, Zhu K, Yuan W, Jiang T, Xue H, Tian J (2022) Fe-do** induced electronic structure reconstruction in Ni-based metal-organic framework for improved energy-saving hydrogen production via urea degradation. J Power Sources 520:230882. https://doi.org/10.1016/j.jpowsour.2021.230882
Liu S, Ma L, Li J (2023) Facile preparation of amorphous NiFe hydroxide by corrosion engineering for electrocatalytic water and urea oxidation. J Alloys Compd 936:168271. https://doi.org/10.1016/j.jallcom.2022.168271
Ding R, Li X, Shi W, Xu Q, Wang L, Jiang H, Yang Z, Liu E (2016) Mesoporous Ni–P nanocatalysts for alkaline urea electrooxidation. Electrochim Acta 222:455–462. https://doi.org/10.1016/j.electacta.2016.10.198
Yang L, Ru F, Shi J, Yang T, Guo C, Chen Y, Wang E, Du Z, Chou K, Hou X (2023) Trifunctional electrocatalysts based on feather-like NiCoP 3D architecture for hydrogen evolution, oxygen evolution, and urea oxidation reactions. Ceram Int 49:659–668. https://doi.org/10.1016/j.ceramint.2022.09.035
Priyadarshi P, Katiyar PK, Maurya R (2022) A review on mechanical, tribological and electrochemical performance of ceramic particle-reinforced Ni-based electrodeposited composite coatings. J Mater Sci 57:19179–19211. https://doi.org/10.1007/s10853-022-07809-1
Ma J, Chi X, Huang Y, Zou R, Li D, Li Z, Li X, Liu C, Peng X (2021) Biomass-based protic ionic liquid derived N, P, co-doped porous carbon-coated CoP nanocrystals for efficient hydrogen evolution reaction. J Mater Sci 56:18188–18199. https://doi.org/10.1007/s10853-021-06235-z
Fang K, Wu T, Hou B, Lin H (2022) Green synthesis of Ni3S2 nanoparticles from a nontoxic sulfur source for urea electrolysis with high catalytic activity. Electrochim Acta 421:140511. https://doi.org/10.1016/j.electacta.2022.140511
Fu Y, Zhang D, Li P, Han Y, You J, Wei Q, Yang W (2023) Tailoring Ni–Fe–Se film on Ni foam via electrodeposition optimization for efficient oxygen evolution reaction. Electrochim Acta 451:142294. https://doi.org/10.1016/j.electacta.2023.142294
Wu JB, **a X, Guo RQ, Huang XH, Lin Y (2017) Ni nanoparticles embedded into cross-linked NiO nanoflakes as enhanced cathode for alkaline batteries. Mater Res Bull 96:315–319. https://doi.org/10.1016/j.materresbull.2017.03.016
Xu Q, Gao W, Wang M, Yuan G, Ren X, Zhao R, Zhao S, Wang Q (2020) Electrodeposition of NiS/Ni2P nanoparticles embedded in amorphous Ni(OH)2 nanosheets as an efficient and durable dual-functional electrocatalyst for overall water splitting. Int J Hydrogen Energy 45:2546–2556. https://doi.org/10.1016/j.ijhydene.2019.11.217
Hu W, Fu H, Chen L, Wu X, Geng B, Huang Y, Xu Y, Du M, Shan G, Song Y, Wu Z, Zheng Q (2023) Synthesis of amorphous Nickel–Cobalt hydroxides with high areal capacitance by one-step electrodeposition using polymeric additive. Chem Eng J 451:138613. https://doi.org/10.1016/j.cej.2022.138613
Li L, Huang W, Lei J, Shang B, Li N, Pan F (2019) Holey nanospheres of amorphous bimetallic phosphide electrodeposited on 3D porous Ni foam for efficient oxygen evolution. Appl Surf Sci 479:540–547. https://doi.org/10.1016/j.apsusc.2019.02.134
Kurowski A, Schultze JW, Staikov G (2002) Initial stages of Ni–P electrodeposition: growth morphology and composition of deposits. Electrochem Commun 4:565–569. https://doi.org/10.1016/S1388-2481(02)00372-7
Wu Y, Zhang Y, Wang Y, He Z, Gu Z, You S (2021) Potentiostatic electrodeposited of Ni–Fe–Sn on Ni foam served as an excellent electrocatalyst for hydrogen evolution reaction. Int J Hydrogen Energy 46:26930–26939. https://doi.org/10.1016/j.ijhydene.2021.05.189
Zhang Y, Chen J, Tan H, Ji H, Li Y (2022) Ni-doped CoP with multi-level hollow structure as efficient electrocatalyst for overall water splitting. J Mater Sci 57:14430–14439. https://doi.org/10.1007/s10853-022-07533-w
Ye Y, Gan Y, Cai R, Dai X, Yin X, Nie F, Ren Z, Wu B, Cao Y, Zhang X (2022) Oxygen vacancies and surface reconstruction on NiFe LDH@Ni(OH)2 heterojunction synergistically triggering oxygen evolution and urea oxidation reaction. J Alloys Compd 921:166145. https://doi.org/10.1016/j.jallcom.2022.166145
Lin S, Yu Y, Sun D, Meng F, Chu W, Huang L, Ren J, Su Q, Ma S, Xu B (2022) FeNi2P three-dimensional oriented nanosheet array bifunctional catalysts with better full water splitting performance than the full noble metal catalysts. J Colloid Interface Sci 608:2192–2202. https://doi.org/10.1016/j.jcis.2021.09.166
Gao Y, Chen Z, Zhao Y, Yu W, Jiang X, He M, Li Z, Ma T, Wu Z, Wang L (2022) Facile synthesis of MoP-Ru2P on porous N, P co-doped carbon for efficiently electrocatalytic hydrogen evolution reaction in full pH range. Appl Catal B-Environ 303:120879. https://doi.org/10.1016/j.apcatb.2021.120879
Wang S, Zhao L, Li J, Tian X, Wu X, Feng L (2022) High valence state of Ni and Mo synergism in NiS2–MoS2 hetero-nanorods catalyst with layered surface structure for urea electrocatalysis. J Energy Chem 66:483–492. https://doi.org/10.1016/j.jechem.2021.08.042
Cao Q, Yuan Y, Wang K, Huang W, Zhao Y, Sun X, Ding R, Lin W, Liu E, Gao P (2022) Phase and crystallinity regulations of Ni(OH)2 by vanadium do** boost electrocatalytic urea oxidation reaction. J Colloid Interface Sci 618:411–418. https://doi.org/10.1016/j.jcis.2022.03.054
Munde AV, Mulik BB, Chavan PP, Sathe BR (2020) Enhanced electrocatalytic activity towards urea oxidation on Ni nanoparticle decorated graphene oxide nanocomposite. Electrochim Acta 349:136386. https://doi.org/10.1016/j.electacta.2020.136386
Elsawy H, Thamer BM, Sedky A, El-Newehy MH (2023) Facile two-step synthesis of nickel nanoparticles supported on 3D porous carbon frameworks as an effective electrocatalyst for urea and methanol oxidation. Mater Chem Phys 297:127361. https://doi.org/10.1016/j.matchemphys.2023.127361
Wang S, Xu P, Tian J, Liu Z, Feng L (2021) Phase structure tuning of graphene supported Ni–NiO Nanoparticles for enhanced urea oxidation performance. Electrochim Acta 370:137755. https://doi.org/10.1016/j.electacta.2021.137755
Lian J, Wu Y, Sun J (2020) High current density electrodeposition of NiFe/Nickel Foam as a bifunctional electrocatalyst for overall water splitting in alkaline electrolyte. J Mater Sci 55:15140–15151. https://doi.org/10.1007/s10853-020-05080-w
Zhang B, Wang S, Ma Z, Qiu Y (2019) Ni0-rich Ni/NiO nanocrystals for efficient water-to-hydrogen conversion via urea electro-oxidation. Appl Surf Sci 496:143710. https://doi.org/10.1016/j.apsusc.2019.143710
Bian H, Chen T, Chen Z, Liu J, Li Z, Du P, Zhou B, Zeng X, Tang J, Liu C (2021) One-step synthesis of mesoporous Cobalt sulfides (CoSx) on the metal substrate as an efficient bifunctional electrode for overall water splitting. Electrochim Acta 389:138786. https://doi.org/10.1016/j.electacta.2021.138786
Yang L, Zhang L (2022) Interfacial electronic modification of bimetallic oxyphosphides as Multi-functional electrocatalyst for water splitting and urea electrolysis. J Colloid Interface Sci 607:546–555. https://doi.org/10.1016/j.jcis.2021.09.013
Li Q, Chen Q, Lei S, Zhai M, Lv G, Cheng M, Xu L, Xu H, Deng Y, Bao J (2023) Crystalline Ni–Fe phosphide/amorphous P doped Fe-(oxy)hydroxide heterostructure as a multifunctional electrocatalyst for solar cell-driven hydrogen production. J Colloid Interface Sci 631:56–65. https://doi.org/10.1016/j.jcis.2022.10.130
Wang F, Zhang K, Zha Q, Ni Y (2022) Honeycomb-like Ni–Mo–S on Ni foam as superior bifunctional electrocatalyst for hydrogen evolution and urea oxidation. J Alloys Compd 899:163346. https://doi.org/10.1016/j.jallcom.2021.163346
Feng J, Chen M, Zhou P, Liu D, Chen Y, He B, Bai H, Liu D, Ip WF, Chen S, Liu D, Feng W, Ni J, Pan H (2022) Reconstruction optimization of distorted FeOOH/Ni hydroxide for enhanced oxygen evolution reaction. Mater Today Energy 27:101005. https://doi.org/10.1016/j.mtener.2022.101005
Li Q, Zheng S, Du M, Pang H (2021) Ultrathin nanosheet metal–organic framework@NiO/Ni nanorod composites. Chem Eng J 417:129201. https://doi.org/10.1016/j.cej.2021.129201
Wei S, Wang X, Wang J, Sun X, Cui L, Yang W, Zheng Y, Liu J (2017) CoS2 nanoneedle array on Ti mesh: a stable and efficient bifunctional electrocatalyst for urea-assisted electrolytic hydrogen production. Electrochim Acta 246:776–782. https://doi.org/10.1016/j.electacta.2017.06.068
Yan L, Sun Y, Hu E, Ning J, Zhong Y, Zhang Z, Hu Y (2019) Facile in-situ growth of Ni2P/Fe2P nanohybrids on Ni foam for highly efficient urea electrolysis. J Colloid Interface Sci 541:279–286. https://doi.org/10.1016/j.jcis.2019.01.096
Acknowledgements
We acknowledge Prof & Head, School of Mechanical and Electrical Engineering, **nxiang University for supporting us by providing lab and technical facilities.
Funding
This work was financially supported by Open Fund of Fujian Provincial Key Laboratory of Functional Materials and Applications (fma2020008), Jiangsu Provincial Double Innovation Program (JSSCBS20211000), the Department Education of Jiangsu Province (No. 23KJB430013), and Guangdong Basic and Applied Basic Research Foundation (2021A1515110092).
Author information
Authors and Affiliations
Contributions
JC: Investigation, writing—original draft preparation, data curation. GW: Investigation, visualization, writing—reviewing and editing. SH: Conceptualization, methodology, project administration, funding acquisition. WY: Analysing and reviewing the results. ZY: Writing—reviewing and editing. YW: Writing—reviewing, editing and funding acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
There is no ethical issues involved in this study.
Additional information
Handling Editor: Pedro Camargo.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cao, J., Wang, G., Hou, S. et al. Facile fabrication of amorphous NiFeP nanosheets to promote urea oxidation reaction for energy-saving hydrogen production. J Mater Sci 58, 16019–16032 (2023). https://doi.org/10.1007/s10853-023-09048-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-023-09048-4