Abstract
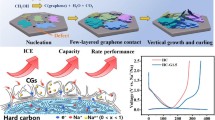
Recently, hard carbons have been extensively studied as anode materials for high-energy rechargeable batteries owing to their low costs, potential high capacities and talented rate capability. Nevertheless, they suffer a low initial Coulombic efficiency (ICE) problem which prohibits their broad practical application. Here we develop a facile prelithiation scheme for hard carbon/graphene (HCG) anodes based on a spontaneous electrochemical reaction with lithium metal foils. The ICE can reach a desirable level by easily tuning the prelithiation time. Importantly, the accurate amount of lithium preloaded into HCG is determined by an atomic adsorption spectrum method. Besides, a similar presodiation process is employed to demonstrate the versatility of this strategy. The surface characterization of prelithiated and presodiated HCG confirms that generated solid electrolyte interface layers have almost identical compositions as those formed during the conventional electrochemical charge–discharge cycles. Moreover, the prelithiated HCG paring with a commercial high-capacity cathode, LiNi0.5Co0.2Mn0.3O2 (NMC), enables the full-cell a comparable galvanostatic capacity and rate capability to NMC half-cell (vs. Li) and a superior cycling performance. These encouraging results indicate an accessible solution to solve problems related to low ICEs of hard carbons.





Similar content being viewed by others
References
Gallego NC, Contescu CI, Meyer HM, Howe JY, Meisner RA, Payzant EA et al (2014) Advanced surface and microstructural characterization of natural graphite anodes for lithium ion batteries. Carbon 72:393–401
Sivakkumar SR, Nerkar JY, Pandolfo AG (2010) Rate capability of graphite materials as negative electrodes in lithium-ion capacitors. Electrochim Acta 55:3330–3335
Dokko K, Nakata N, Suzuki Y, Kanamura K (2010) High-rate lithium deintercalation from lithiated graphite single-particle electrode. J Phys Chem C 114:8646–8650
Yu ZL, **n S, You Y, Yu L, Lin Y, Xu DW et al (2016) Ion-catalyzed synthesis of microporous hard carbon embedded with expanded nanographite for enhanced lithium/sodium storage. J Am Chem Soc 138:14915–14922
**ao L, Cao Y, Henderson WA, Sushko ML, Shao Y, **ao J et al (2016) Hard carbon nanoparticles as high-capacity, high-stability anodic materials for Na-ion batteries. Nano Energy 19:279–288
Bommier C, Leonard D, Jian ZL, Stickle WF, Greaney PA, Ji XL (2016) New paradigms on the nature of solid electrolyte interphase formation and capacity fading of hard carbon anodes in Na-Ion batteries. Adv Mater Interfaces 3:1600449
Slater MD, Kim D, Lee E, Johnson CS (2013) Sodium-Ion batteries. Adv Funct Mater 23:947–958
Liu Y, Xue JS, Zheng T, Dahn JR (1996) Mechanism of lithium insertion in hard carbons prepared by pyrolysis of epoxy resins. Carbon 34:193–200
Dahn JR, **ng W, Gao Y (1997) The “falling cards model” for the structure of microporous carbons. Carbon 35:825–830
Wang J, Liu J-L, Wang Y-G, Wang C-X, **a Y-Y (2012) Pitch modified hard carbons as negative materials for lithium-ion batteries. Electrochim Acta 74:1–7
Zhu H, Jia Z, Chen Y, Weadock N, Wan J, Vaaland O et al (2013) Tin anode for sodium-ion batteries using natural wood fiber as a mechanical buffer and electrolyte reservoir. Nano Lett 13:3093–3100
Prabakar SJR, Jeong J, Pyo M (2015) Nanoporous hard carbon anodes for improved electrochemical performance in sodium ion batteries. Electrochim Acta 161:23–31
Yang R, Wang Z, Liu J, Chen L (2004) Nano Co3O4 particles embedded in porous hard carbon spherules as anode material for Li-Ion batteries. Electrochem Solid-State Lett 7:A496–A499
Zhu GN, Du YJ, Wang YG, Yu AS, **a YY (2013) Electrochemical profile of lithium titanate/hard carbon composite as anode material for Li-ion batteries. J Electroanal Chem 688:86–92
Kim C, Choi S, Yoo S, Kwon D, Ko S, Kim J-M et al (2015) A facile route for growth of CNTs on Si@hard carbon for conductive agent incorporating anodes for lithium-ion batteries. Nanoscale 7:11286–11290
Guo B, Shu J, Wang Z, Yang H, Shi L, Liu Y et al (2008) Electrochemical reduction of nano-SiO2 in hard carbon as anode material for lithium ion batteries. Electrochem Commun 10:1876–1878
Li YM, Hu YS, Titirici MM, Chen LQ, Huang XJ (2016) Hard carbon microtubes made from renewable cotton as high-performance anode material for sodium-ion batteries. Adv Energy Mater 6:1600659–n/a
Li W, Zeng L, Yang Z, Gu L, Wang J, Liu X et al (2014) Free-standing and binder-free sodium-ion electrodes with ultralong cycle life and high rate performance based on porous carbon nanofibers. Nanoscale 6:693–698
Guerin K, Fevrier-Bouvier A, Flandrois S, Simon B, Biensan P (2000) On the irreversible capacities of disordered carbons in lithium-ion rechargeable batteries. Electrochim Acta 45:1607–1615
Béguin F, Chevallier F, Vix-Guterl C, Saadallah S, Bertagna V, Rouzaud JN et al (2005) Correlation of the irreversible lithium capacity with the active surface area of modified carbons. Carbon 43:2160–2167
Jarvis CR, Lain MJ, Yakovleva MV, Gao Y (2006) A prelithiated carbon anode for lithium-ion battery applications. J Power Sources 162:800–802
Cao WJ, Zheng JP (2012) Li-ion capacitors with carbon cathode and hard carbon/stabilized lithium metal powder anode electrodes. J Power Sources 213:180–185
Wang Z, Fu Y, Zhang Z, Yuan S, Amine K, Battaglia V et al (2014) Application of Stabilized Lithium Metal Powder (SLMP®) in graphite anode—a high efficient prelithiation method for lithium-ion batteries. J Power Sources 260:57–61
Zhao H, Wang Z, Lu P, Jiang M, Shi F, Song X et al (2014) Toward practical application of functional conductive polymer binder for a high-energy lithium-ion battery design. Nano Lett 14:6704–6710
Liu N, Hu L, McDowell MT, Jackson A, Cui Y (2011) Prelithiated silicon nanowires as an anode for lithium ion batteries. ACS Nano 5:6487–6493
Kim HJ, Choi S, Lee SJ, Seo MW, Lee JG, Deniz E et al (2016) Controlled prelithiation of silicon monoxide for high performance Lithium-Ion rechargeable full cells. Nano Lett 16:282–288
Zhou H, Wang X, Chen D (2015) Li-metal-free prelithiation of Si-based negative electrodes for full Li-ion batteries. Chemsuschem 8:2737–2744
Cao Z, Xu P, Zhai H, Du S, Mandal J, Dontigny M et al (2016) Ambient-air stable lithiated anode for rechargeable Li-Ion batteries with high energy density. Nano Lett 16:7235–7240
Wu SC, Zhu K, Tang J, Liao KM, Bai SY, Yi J et al (2016) A long-life lithium ion oxygen battery based on commercial silicon particles as the anode. Energ Environ Sci 9:3262–3271
Zhang X, Han SC, **ao PG, Fan CL, Zhang WH (2016) Thermal reduction of graphene oxide mixed with hard carbon and their high performance as lithium ion battery anode. Carbon 100:600–607
Komaba S, Ishikawa T, Yabuuchi N, Murata W, Ito A, Ohsawa Y (2011) Fluorinated ethylene carbonate as electrolyte additive for rechargeable Na batteries. ACS Appl Mater Interfaces 3:4165–4168
Komaba S, Murata W, Ishikawa T, Yabuuchi N, Ozeki T, Nakayama T et al (2011) Electrochemical Na insertion and solid electrolyte interphase for hard-carbon electrodes and application to Na-Ion batteries. Adv Funct Mater 21:3859–3867
Lu M, Cheng H, Yang Y (2008) A comparison of solid electrolyte interphase (SEI) on the artificial graphite anode of the aged and cycled commercial lithium ion cells. Electrochim Acta 53:3539–3546
Zheng Y, He Y-B, Qian K, Li B, Wang X, Li J et al (2015) Deterioration of lithium iron phosphate/graphite power batteries under high-rate discharge cycling. Electrochim Acta 176:270–279
Kang SH, Abraham DP, **ao A, Lucht BL (2008) Investigating the solid electrolyte interphase using binder-free graphite electrodes. J Power Sources 175:526–532
Eshkenazi V, Peled E, Burstein L, Golodnitsky D (2004) XPS analysis of the SEI formed on carbonaceous materials. Solid State Ionics 170:83–91
Aurbach D, Gamolsky K, Markovsky B, Gofer Y, Schmidt M, Heider U (2002) On the use of vinylene carbonate (VC) as an additive to electrolyte solutions for Li-ion batteries. Electrochim Acta 47:1423–1439
Lindgren F, Xu C, Niedzicki L, Marcinek M, Gustafsson T, Bjorefors F et al (2016) SEI formation and interfacial stability of a Si electrode in a LiTDI-salt based electrolyte with FEC and VC additives for Li-Ion batteries. ACS Appl Mater Interfaces 8:15758–15766
Wang Y-S, Huang C-M, Hsieh H-W, Lin Y-F, Lin C-Y, Lee J-T (2014) Effect of temperature on the dissolution of solid electrolyte interface on mesocarbon microbeads electrodes in propylene carbonate-based electrolytes. Electrochim Acta 142:34–42
Brisson PY, Darmstadt H, Fafard M, Adnot A, Servant G, Soucy G (2006) X-ray photoelectron spectroscopy study of sodium reactions in carbon cathode blocks of aluminium oxide reduction cells. Carbon 44:1438–1447
Yang X, Zuo Z, Wang H, Chen Q, Zhang H, Huang Z et al (2015) The contradiction between the half-cell and full-battery evaluations on the Tungsten-coating LiNi0.5Co0.2Mn0.3O2 cathode. Electrochim Acta 180:604–609
Noh M, Cho J (2013) Optimized synthetic conditions of LiNi0.5Co0.2Mn0.3O2 cathode materials for high rate lithium batteries via co-precipitation method. J Electrochem Soc 160:A105–A111
Acknowledgements
This work was supported by the National Science Foundation of China (Grant No. 51372079, No. 51472082 and No. 51672079) and the Graduate Student Research Innovation Project of Hunan Province (CX2015B065).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, X., Fan, C. & Han, S. Improving the initial Coulombic efficiency of hard carbon-based anode for rechargeable batteries with high energy density. J Mater Sci 52, 10418–10430 (2017). https://doi.org/10.1007/s10853-017-1206-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1206-3