Abstract
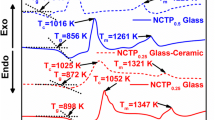
Glass samples with general formula NaCo1−x (VO) x PO4 (x = 0.1, 0.3, 0.5 and 0.7) are synthesized via a simple melt quenching method followed by high-energy ball milling for 30 h to form the homogeneous nanoscaled glass powders. DTA traces of all the glass and glass–ceramic samples indicated exothermic processes confirming selective crystallization induced in the glass network. The formation of major crystalline phase [sodium cobalt pyrophosphate (Na2CoP2O7)] with an ordered layered structure was monitored by X-ray diffraction and the same was justified by SEM images. Structural illustration of major crystalline Na2CoP2O7 phase offered more intra-layer Co–Co distance (7.12 Å) than inter-layer Co–Co distance (5.37 Å) which facilitates two-dimensional Na-ion diffusion pathways to achieve the fast intercalation and de-intercalation phenomenon along the a and c directions. The ionic conductivity was monitored by Impedance analysis and achieved to be highest (6.41 × 10−7 S cm−1) for the glass–ceramic cathode x = 0.3, NaCo1−x (VO) x PO4. The initial discharge capacity for the highest conducting NaCo0.7(VO)0.3PO4 cathode is obtained as 93 mA h g−1 in 0.1 C and had 65% capacity retention even at high rate 10 C.








Similar content being viewed by others
References
Nitta N, Wu F, Lee JT, Yushin G (2015) Li-ion battery materials: present and future. Mater Today 18(5):252–264
Takada K (2013) Progress and prospective of solid-state lithium batteries. Acta Mater 61(3):759–770
Tarascon JM (2010) Key challenges in future Li-battery research. Philos Trans R Soc A 368(1923):3227–3241
Palomares V, Serras P, Villaluenga I, Hueso KB, Carretero-González J, Rojo T (2012) Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ Sci 5(3):5884–5901
Pan H, Hu YS, Chen L (2013) Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ Sci 6(8):2338–2360
Ma X, Chen H, Ceder G (2011) Electrochemical properties of monoclinic NaMnO2. J Electrochem Soc 158(12):A1307–A1312
Johnson C (2016). Synthesis and evaluation of NaMPO4 (M = Fe, Mn, Co) framework polyanion cathodes for sodium-ion batteries (SIB). In: 18th international meeting on lithium batteries, Ecs, 19–24 June
Zaghib K, Trottier J, Hovington P, Brochu F, Guerfi A, Mauger A, Julien CM (2011) Characterization of Na-based phosphate as electrode materials for electrochemical cells. J Power Sources 196(22):9612–9617
Zhu Y, Xu Y, Liu Y, Luo C, Wang C (2013) Comparison of electrochemical performances of olivine NaFePO4 in sodium-ion batteries and olivine LiFePO4 in lithium-ion batteries. Nanoscale 5(2):780–787
Li ZY, Zhang J, Gao R, Zhang H, Hu Z, Liu X (2016) Unveiling the role of Co in improving the high-rate capability and cycling performance of layered Na0.7Mn0. 7Ni0.3-xCoxO2 cathode materials for sodium ion batteries. ACS Appl Mater Interfaces. doi:10.1021/acsami.6b04073
Amine K, Yasuda H, Yamachi M (2000) Olivine LiCoPO4 as 4.8 V electrode material for lithium batteries. Electrochem Solid State Lett 3(4):178–179
Okada S, Sawa SI, Uebou Y, Egashira M, Yamaki JI, Tabuchi M, Kageyama H (2003) Charge-discharge mechanism of LiCoPO4 cathode for rechargeable lithium batteries. Electrochemistry 71(12):1136–1138
Xu J, Lee DH, Clément RJ, Yu X, Leskes M, Pell AJ, Meng YS (2014) Identifying the critical role of Li substitution in P2–Nax[LiyNizMn1–y–z] O2 (0 < x, y, z < 1) intercalation cathode materials for high-energy Na-ion batteries. Chem Mater 26(2):1260–1269
Chen CY, Matsumoto K, Nohira T, Hagiwara R (2014) Na2MnSiO4 as a positive electrode material for sodium secondary batteries using an ionic liquid electrolyte. Electrochem Commun 45:63–66
Kercher AK, Ramey JO, Carroll KJ, Kiggans JO, Dudney NJ, Meisner RA, Veith GM (2014) Mixed polyanion glass cathodes: iron phosphate vanadate glasses. J Electrochem Soc 161(14):A2210–A2215
Kercher AK, Kolopus JA, Carroll KJ, Unocic RR, Kirklin S, Wolverton C, Dudney NJ (2016) Mixed polyanion glass cathodes: glass-state conversion reactions. J Electrochem Soc 163(2):A131–A137
Aoyagi T, Fujieda T, Mitsuishi K, Kawaji J, Toyama T, Kono K, Naito T (2014) V2O5-P2O5-Fe2O3-Li2O glass-ceramics as high-capacity cathode for lithium-ion batteries. Mater Res Soc Symp Proc 1643:13–1643. doi:10.1557/opl.2014.246
Barpanda P, Ye T, Nishimura SI, Chung SC, Yamada Y, Okubo M, Yamada A (2012) Sodium iron pyrophosphate: a novel 3.0 V iron-based cathode for sodium-ion batteries. J Electrochem Commun 24:116–119
Murugan GS, Varma KBR (2002) Lithium borate–strontium bismuth tantalate glass nanocomposite: a novel material for nonlinear optic and ferroelectric applications. J Mater Chem 12(5):1426–1436
Delaizir G, Seznec V, Rozier P, Surcin C, Salles P, Dolle M (2013) Electrochemical performances of vitreous materials in the system Li2O–V2O5–P2O5 as electrode for lithium batteries. Solid State Ionics 237:22–27
Hassaan MY, Salem SM, Moustafa MG (2014) Study of nanostructure and ionic conductivity of Li1.3Nb0.3V1.7(PO4)3 glass ceramics used as cathode material for solid batteries. J Non-Cryst Solids 391:6–11
Rietveld H (1969) A profile refinement method for nuclear and magnetic structures. J Appl Crystallogr 2(2):65–71
Rietveld HM (1967) Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Crystallogr 22(1):151–152
Erragh F, Boukhari A, Elouadi B, Holt EM (1991) Crystal structures of two allotropic forms of Na2CoP2O7. J Cryst Spectrosc 21(3):321–326
Sanz F, Parada C, Rojo JM, Ruiz-Valero C, Saez-Puche R (1999) Studies on tetragonal Na2CoP2O7, a novel ionic conductor. J Solid State Chem 145(2):604–611
Williamson GK, Hall WH (1953) X-ray line broadening from filed aluminium and wolfram. Acta Metall Mater 1(1):22–31
Momma K, Izumi F (2011) VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J Appl Crystallogr 44(6):1272–1276
Honma T, Sato A, Ito N, Togashi T, Shinozaki K, Komatsu T (2014) Crystallization behavior of sodium iron phosphate glass Na2-xFe1+0.5xP2O7 for sodium ion batteries. J Non-Cryst Solids 404:26–31
Chowdari BVR, Rao GS, Lee GYH (2000) XPS and ionic conductivity studies on Li2O–Al2O3–(TiO2 or GeO2)–P2O5 glass–ceramics. Solid State Ionics 136:1067–1075
Zhang Q, Wen Z, Liu Y, Song S, Wu X (2009) Na+ ion conductors of glass–ceramics in the system Na1+xAlxGe2−xP3O12 (0.3 ≤ x ≤ 1.0). J Alloys Compd 479(1):494–499
Afyon S, Krumeich F, Mensing C, Borgschulte A, Nesper R (2014) New high capacity cathode materials for rechargeable Li-ion batteries: vanadate-borate glasses. Sci Rep 4:7113. doi:10.1038/srep07
Acknowledgements
This work has been funded by NAVAL RESEARCH BOARD, DRDO, Govt. of INDIA, Grant No: NRB-311/MAT/13-14. The authors thank Dr. S.K. Martha, Department of Chemistry, IIT Hyderabad for his kind help in acquiring CV studies and discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R. Balaji Rao has received Grants from NAVAL RESEARCH BOARD, DRDO, Govt. of INDIA, Grant No: NRB-311/MAT/13-14.
Rights and permissions
About this article
Cite this article
Suman, G., Rao, C.S., Ojha, P.K. et al. Mixed polyanion NaCo1−x (VO) x PO4 glass–ceramic cathode: role of ‘Co’ on structural behaviour and electrochemical performance. J Mater Sci 52, 5038–5047 (2017). https://doi.org/10.1007/s10853-016-0741-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0741-7