Abstract
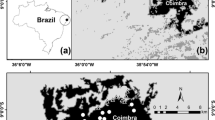
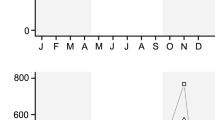
For butterflies, tolerance to the matrix may be an important criterion of habitat occurrence in fragmented landscapes. Here we examine the relative effects of habitat fragmentation and the surrounding agricultural matrix on the functional composition of fruit-feeding butterflies of the Atlantic rain forest in southeastern Brazil. Generalized linear models were used to detect the effects of landscape metrics on butterfly richness and abundance of the total assemblage and functional groups. Circular statistics were used to analyze the patterns of monthly abundance of the total assemblage and functional groups in the forest remnants and the surrounding matrices. In total, 650 butterflies representing 57 species were captured; species composition differed significantly between the forest fragments and the surrounding matrices. We recorded 22 forest specialists, 18 matrix specialists, 11 common species with matrix preference and six common species with forest preference. Forest connectivity favored the richness of forest specialists, while habitat fragmentation enhances the richness and abundance of matrix-tolerant species. Circular analysis revealed that forest specialists were more abundant in the rainy season while matrix-tolerant species proliferated in the dry season. Although maintaining connectivity of forest fragments may increase the mobility and dispersion of forest species, our results showed that landscape fragmentation modify butterfly assemblage by promoting an increase of matrix tolerant species with detriment of forest specialists.






Similar content being viewed by others
References
Batschelet E (1981) Circular statistics in biology. Academic Press, London
Beccaloni GW, Hall SK, Viloria AL, Robinson GS (2008) Catalogue of the hostplants of the Neotropical butterflies/Catálogo de las plantas huésped de las mariposas Neotropicales. In: Ribescyted SEA (ed) Monografias Tercer Milenio, vol 8. The Natural History Museum, Instituto Venezolano de Investigaciones Científica, Zaragoza
Bender D, Fahrig L (2005) Matrix structure obscures the relationship between interpatch movement and patch size and isolation. Ecology 86:1023–1033. doi:10.1890/03-0769
Brower LP (1996) Monarch butterfly orientation: missing pieces of a magnificent puzzle. J Exp Biol 199:93–103
Brown KS Jr (1992) Borboletas da Serra do Japi: diversidade, habitats, recursos alimentares e variação temporal. In: Morellato LPC (ed) História Natural da Serra do Japi: Ecologia e Preservação de uma área Florestal no Sudeste do Brasil. Editora Unicamp, Campinas, pp 142–186
Brown KS Jr, Brown GG (1992) Habitat alteration and species loss in Brazilian forests. In: Whitmore TC, Sayer JA (eds) Tropical deforestation and species extinctions. Chapman and Hall, Londres, pp 119–140
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Clarke KR, Green RH (1988) Statistical design and analysis for a ‘biological effects’ study. Mar Ecol Prog Ser 46:213–226. doi:10.3354/meps046213
Colwell RK (2013) EstimateS: statistical estimation of species richness and shared species from samples. Version 9. http://purloclc.org/estimates. Accessed 22 Nov 2013
Costa CMR (1998) Biodiversidade em Minas Gerais: um atlas para a sua conservação. Fundação Biodiversitas, Belo Horizonte
De’ath G, Fabricius E (2000) Classification and regression trees: a powerful yet simple technique for ecological data analysis. Ecology 81:3178–3192. doi:10.1890/0012-9658(2000)081[3178:CARTAP]2.0.CO;2
Dennis RLH, Hardy PB (2007) Support for mending the matrix: resource seeking by butterflies in apparent non-resource zones. J Insect Conserv 11:157–168. doi:10.1007/s10841-006-9032-y
Devries PJ (1987) The butterflies of Costa Rica and their natural history: Papilionidae, Pieridae, and Nymphalidae. Princeton University Press, Princeton
Didham RK, Ghazoul J, Stork NE, Davis AJ (1996) Insects in fragmented forests: a functional approach. Trends Ecol Evol 11:255–260. doi:10.1016/0169-5347(96)20047-3
Dormann CF, McPherson JM, Araujo MB, Bivand R, Bolliger J, Carl G, Davies RG, Hirzel A, Jetz W, Kissling WD, Kuhn I, Ohlemuller R, Peres-Neto PR, Reineking B, Schroder B, Schurr FM, Wilson R (2007) Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography 30:609–628. doi:10.1111/j.2007.0906-7590.05171.x
Dover J, Settele J (2009) The influences of landscape structure on butterfly distribution and movement: a review. J Insect Conserv 13:3–27. doi:10.1007/s10841-008-9135-8
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515. doi:10.1146/annurev.ecolsys.34.011802.132419
Fahrig L (2007) Non-optimal animal movement in human-altered landscapes. Funct Ecol 21:1003–1015. doi:10.1111/j.1365-2435.2007.01326.x
Foley JA, DeFries R, Asner GP, Barford C, Bonan G, Carpenter SR, Chapin FS, Coe MT, Daily GC, Gibbs HK, Helkowski JH, Holloway T, Howard EA, Kucharik CJ, Monfreda C, Patz JA, Prentice IC, Ramankutty N, Snyder PK (2005) Global consequences of land use. Science 309:570–574. doi:10.1126/science.1111772
Freitas AVL, Brown KS Jr (2004) Phylogeny of the Nymphalidae (Lepidoptera). Syst Biol 53:363–383. doi:10.1080/10635150490445670
Fundação SOS Mata Atlântica, Instituto Nacional de Pesquisas Espaciais (2009) Atlas dos remanescentes florestais da Mata Atlântica, período de 2005–2008. Fundação SOS Mata Atlântica & São Jose dos Campos, INPE, São Paulo
Hamer KC, Hill JK, Mustaffa N, Benedick S, Sherratt TN, Chey VK, Maryati M (2005) Temporal variation in abundance and diversity of butterflies in Bornean rain forests: opposite impacts of logging recorded in different seasons. J Trop Ecol 21:1–9. doi:10.1017/S0266467405002361
Hamer KC, Hill JK, Benedick S, Mustaffa N, Chey VK, Mohamed M (2006) Diversity and ecology of carrion and fruit-feeding butterflies in Bornean rainforest. J Trop Ecol 22:25–33. doi:10.1017/S0266467405002750
Hammer O, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:1–9
Hanski I (1999) Metapopulation ecology. Oxford University Press, Oxford
Hogue CL (1993) Latin American: insects and entomology. University of California Press, Berkeley
IBGE (2004) Mapa de biomas do Brasil. Escala 1:5.000.000. Rio de Janeiro. http://mapas.ibge.gov.br/biomas2/viewer.htm. Accessed 15 Aug 2011
Jokimaki J, Huhta E (1996) Effects of landscape matrix and habitat structure on a bird community in northern Finland: a multi-scale approach. Ornis Fenn 73:97–113
Knowlton JL, Graham CH (2010) Using behavioral landscape ecology to predict species’ responses to land use and climate change. Biol Conserv 143:1342–1354. doi:10.1016/j.biocon.2010.03.011
Kovach WL (2010) Oriana for Windows, version 3.0. Kovach Computer Services, Pentraeth
Laurance WF, Delamonica P, Laurance SG, Vasconcelos HL, Lovejoy TE (2000) Rainforest fragmentation kills big trees. Nature 404:836. doi:10.1038/35009032
Laurance WF, Nascimento HEM, Laurance SG, Andrade AC, Fearnside F, Ribeiro JELS, Capretz RL (2006) Rain forest fragmentation and the proliferation of successional trees. Ecology 87:469–482. doi:10.1890/05-0064
Lomolino M, Perault D (2001) Island biogeography and landscape ecology of mammals inhabiting fragmented, temperate rain forests. Glob Ecol Biogeogr 10:113–132. doi:10.1046/j.1466-822x.2001.00221.x
Lopes AV, Girão LC, Santos BA, Peres CA, Tabarelli M (2009) Long-term erosion of tree reproductive trait diversity in edge-dominated Atlantic forest fragments. Biol Conserv 142:1154–1165. doi:10.1016/j.biocon.2009.01.007
Marini-Filho OJ, Martins RP (2010) Nymphalid butterfly dispersal among forest fragments at Serra da Canastra National Park, Brazil. J Insect Conserv 14:401–411. doi:10.1007/s10841-010-9271-9
McIntire EJB, Schultz CB, Crone EE (2007) Designing a network for butterfly habitat restoration: where individuals, populations and landscapes interact. J Appl Ecol 44:725–736. doi:10.1111/j.1365-2664.2007.01326.x
Molleman F, Kop A, Brakefield PM, DeVries PJ, Zwaan BS (2006) Vertical and temporal patterns of biodiversity of fruit-feeding butterflies in a tropical forest in Uganda. Biodivers Conserv 15:107–121. doi:10.1007/s10531-004-3955-y
Morellato LPC, Haddad CFB (2000) Introduction: the Brazilian Atlantic forest. Biotropica 32:786–792. doi:10.1111/j.1744-7429.2000.tb00618.x
Morellato LPC, Talora DC, Takahasi A, Bencke CC, Romera EC, Zipparro VB (2000) Phenology of Atlantic rain forest trees: a comparative study. Biotropica 32:811–823. doi:10.1111/j.1744-7429.2000.tb00620.x
Morellato LPC, Alberti LF, Hudson IL (2010) Applications of circular statistics in plant phenology: a case studies approach. In: Hudson IL, Keatley MR (eds) Phenological research: methods for environmental and climate change analysis. Springer, Berlin, pp 339–359
Munguira ML, García-Barros E, Cano MJ (2009) Butterfly herbivory and larval ecology. In: Settele J, Shreeve T, Konvička M, Van Dyck H (eds) Ecology of butterflies in Europe. Cambridge University Press, Cambridge, pp 43–54
Murakami M, Ichie T, Hirao T (2008) Beta-diversity of lepidopteran larval communities in a Japanese temperate forest: effects of phenology and tree species. Ecol Res 23:179–187. doi:10.1007/s11284-007-0353-4
Novotny V, Basset Y (1998) Seasonality of sap-sucking insects (Auchenorrhyncha, Hemiptera) feeding on Ficus (Moraceae) in a lowland rain forest in New Guinea. Oecologia 115:514–522
Novotny V, Miller SE, Leps J, Basset Y, Bito D, Janda M, Hulcr J, Damas K, Weiblen GD (2004) No tree an island: the plant–caterpillar food web of a secondary rain forest in New Guinea. Ecol Lett 7:1090–1100
Otero LS (1971) Insetos brasileiros e seu meio. Koyo Shoin Comp. Ltda, Tokyo
Peña C, Wahlberg N (2008) Prehistorical climate change increased diversification of a group of butterflies. Biol Lett 4:274–278. doi:10.1098/rsbl.2008.0062
Perfecto I, Vandermeer J (2002) Quality of agroecological matrix in a tropical montane landscape: ants in coffee plantations in southern Mexico. Conserv Biol 16:174–182. doi:10.1046/j.1523-1739.2002.99536.x
Prugh LR, Hodges KE, Sinclair RE, Brashares JS (2008) Effect of habitat area and isolation on fragmented animal populations. Proc Natl Acad Sci USA 105:20770–20775. doi:10.1073/pnas.0806080105
Ramos FN, Santos FAM (2005) Phenology of Psychotria tenuinervis (Rubiaceae) in Atlantic forest fragments. Can J Bot 83:1305–1316. doi:10.1139/b05-106
Ribeiro DB, Freitas AVL (2011) Large-sized insects show stronger seasonality than small-sized ones: a case study of fruit-feeding butterflies. Biol J Linn Soc 104:820–827
Ribeiro DB, Prado PI, Brown KS Jr, Freitas AVL (2010) Temporal diversity patterns and phenology in fruit-feeding butterflies in the Atlantic forest. Biotropica 42:710–716. doi:10.1111/j.1744-7429.2010.00648.x
Ribeiro DB, Batista R, Prado PI, Brown KS Jr, Freitas AV (2012) The importance of small scales to the fruit-feeding butterfly assemblages in a fragmented landscape. Biodivers Conserv 21:811–827. doi:10.1007/s10531-011-0222-x
Ricketts NT (2001) The matrix matters: effective isolation in fragmented landscapes. Am Nat 158:87–99. doi:10.1086/320863
Ries L, Debinski DM (2001) Butterfly responses to habitat edges in the highly fragmented prairies of Central Iowa. J Anim Ecol 70:840–852. doi:10.1046/j.0021-8790.2001.00546.x
Rosch V, Tscharntke T, Scherber C, Batáry P (2013) Landscape composition, connectivity and fragment size drive effects of grassland fragmentation on insect communities. J App Ecol 50:387–394. doi:10.1111/1365-2664.12056
Saunders DA, Hobbs RJ, Margules CR (1991) Biological consequences of ecosystem fragmentation: a review. Conserv Biol 5:18–32. doi:10.1111/j.1523-1739.1991.tb00384.x
Shuey JA (1997) An optimizing portable bait trap for quantitative sampling of butterflies. Trop Lepid 8:1–4
Uehara-Prado M, Brown KS, Freitas AVL (2007) Species richness, composition and abundance of fruitfeeding butterflies in the Brazilian Atlantic forest: comparison between a fragmented and a continuous landscape. Glob Ecol Biogeogr 16:43–54. doi:10.1111/j.1466-8238.2006.00267.x
Umetsu F, Metzger JP, Pardini R (2008) Importance of estimating matrix quality for modeling species distribution in complex tropical landscapes: a test with Atlantic forest small mammals. Ecography 31:359–370. doi:10.1111/j.0906-7590.2008.05302.x
Veech JA, Summerville KS, Crist TO, Gering JC (2002) The additive partitioning of diversity: recent revival of an old idea. Oikos 99:3–9. doi:10.1034/j.1600-0706.2002.990101.x
Vos CC, Berry P, Opdam P, Baveco H, Nijhof B, O’Hanley J, Bell C, Kuipers H (2008) Adapting landscapes to climate change: examples of climate-proof ecosystem networks and priority adaptation zones. J Appl Ecol 45:1722–1731. doi:10.1111/j.1365-2664.2008.01569.x
Wahlberg N, Leneveu J, Kodandaramaiah U, Pena C, Nylin S, Freitas AVL, Brower AVZ (2009) Nymphalid butterflies diversify following near demise at the cretaceous/tertiary boundary. Proc R Soc Lond B Biol Sci 276:4295–4302
Walla TR, Engen S, DeVries PJ, Lande R (2004) Modeling vertical beta-diversity in tropical butterfly communities. Oikos 107:610–618. doi:10.1111/j.0030-1299.2004.13371.x
Watling JI, Nowakowski AJ, Donnelly MA, Orrock JL (2011) Meta-analysis reveals the importance of matrix composition for animals in fragmented habitat. Grob Ecol Biogeogr 20:209–217. doi:10.1111/j.1466-8238.2010.00586.x
Wirth R, Meyer ST, Leal IR, Tabarelli M (2008) Plant–herbivore interactions at the forest edge. Prog Bot 68:423–448. doi:10.1007/978-3-540-72954-9_17
Wolda H (1989) Seasonal cues in tropical organisms: rainfall? Not necessarily! Oecologia 80:437–442. doi:10.1007/BF00380064
Zar JH (1996) Biostatistical analysis. Prentice Hall, Englewood Cliffs
Acknowledgments
We thank André Freitas for their great help in identifying the butterflies and making possible the access to the collections of the University of Campinas (Unicamp). Karina L. Silva-Brandão, Martin Pareja and the anonymous referee made valuable suggestions in the manuscript. We also thank Alexandre M. Dos Santos (PIBICT/Fapemig) and Eduardo Loureiro Abreu who helped throughout the field work. This research was financially supported by a grant from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior to M. Monteiro. The project was supported by FAPEMIG/Vale Company (RDP-00104-10), as well as CNPq (472250/2010-8).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Brito, M.M., Ribeiro, D.B., Raniero, M. et al. Functional composition and phenology of fruit-feeding butterflies in a fragmented landscape: variation of seasonality between habitat specialists. J Insect Conserv 18, 547–560 (2014). https://doi.org/10.1007/s10841-014-9650-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-014-9650-8