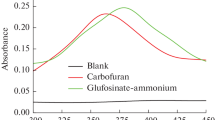
A method based on the inhibition of plant esterase from pesticides has been proposed and validated for the determination of carbendazim residues in aqueous samples. After optimization in pH, temperature, and detection time, a lower detection limit of 0.105 μM was obtained in the linear range from 0.105 to 41.84 μM. Upon analysis of the detection mechanism, carbendazim was found to be binding to plant esterase via the van der Waals force and the hydrogen bond with a single binding site through an exothermic reaction. Moreover, the proposed method was satisfactorily applied to the analysis of carbendazim residues in different aqueous samples, obtaining a remarkably good agreement with the standardized reference method.
Similar content being viewed by others
References
L. Pareja, A. R. Fernandez-Alba, V. Cesio, and H. Heinzen, Trac-Trend. Anal. Chem., 30, 270–291 (2011).
V. Janaki Devi, N. Nagarani, M. Yokesh Babu, A. K. Kumaraguru, and C. M. Ramakritinan, Chemosphere, 90, 1158–1166 (2013).
M. Asensio-Ramos, J. Hernandez-Borges, G. Gonzalez-Hernandez, and M. Angel Rodriguez-Delgado, Electrophoresis, 33, 2184–2191 (2012).
A. Moral, M. Dolores Sicilia, and S. Rubio, Anal. Chim. Acta, 650, 207–213 (2009).
C. J. Sinclair, A. B. A. Boxall, S. A. Parsons, and M. R. Thomas, Environ. Sci. Technol., 40, 7283–7289 (2006).
S. C. Utture, K. Banerjee, S. Dasgupta, S. H. Patil, M. R. Jadhav, S. S. Wagh, S. S. Kolekar, M. A. Anuse, and P. G. Adsule, J. Agric. Food Chem., 59, 7866–7873 (2011).
M. del Pozo, M. Alonso, L. Hernandez, and C. Quintana, Electroanalysis, 23, 189–195 (2011).
A. Nougadere, V. Sirot, A. Kadar, A. Fastier, E. Truchot, C. Vergnet, F. Hommet, J. Bayle, P. Gros, and J.-C. Leblanc, Environ. Int., 45, 135–150 (2012).
Q. Subhani, Z. Huang, Z. Zhu, and Y. Zhu, Talanta, 116, 127–132 (2013).
M. J. Jonker, A. M. Piskiewicz, N. Ivorra, and J. E. Kammenga, Environ. Toxicol. Chem., 23, 1529–1537 (2004).
J. Dominguez-Alvarez, M. Mateos-Vivas, D. Garcia-Gomez, E. Rodriguez-Gonzalo, and R. Carabias-Martinez, J. Chromatogr. A, 1278, 166–174 (2013).
M. del Pozo, L. Hernandez, and C. Quintana, Talanta, 81, 1542–1546 (2010).
S. Luo, Y. Wu, and H. Gou, Ionics, 19, 673–680 (2013).
Eurosurveillance editorial, Eur. Communic. Dis. Bull., 15, 19641 (2010).
M. J. Rodriguez-Cuesta, R. Boque, F. X. Rius, D. P. Zamora, M. M. Galera, and A. G. Frenich, Anal. Chim. Acta, 491, 47–56 (2003).
J. F. G. Reyes, P. O. Barrales, and A. M. Diaz, Anal. Chim. Acta, 493, 35–45 (2003).
C. L. da Silva, E. C. de Lima, and M. F. M. Tavares, J. Chromatogr. A, 1014, 109–116 (2003).
K. P. Prousalis, D. A. Polygenis, A. Syrokou, F. N. Lamari, and T. Tsegenidis, Anal. Bioanal. Chem., 379, 458–463 (2004).
C. Lesueur, M. Gartner, A. Mentler, and M. Fuerhacker, Talanta, 75, 284–293 (2008).
R. Halko, C. P. Sanz, Z. S. Ferrera, and J. J. S. Rodriguez, Chromatographia, 60, 151–156 (2004).
E. Rodriguez-Gonzalo, J. Dominguez-Alvarez, L. Ruano-Miguel, and R. Carabias-Martinez, Electrophoresis, 29, 4066–4077 (2008).
S. B. Singh, G. D. Foster, and S. U. Khan, J. Chromatogr. A, 1148, 152–157 (2007).
A. D. Strickland and C. A. Batt, Anal. Chem., 81, 2895–2903 (2009).
Y. Guo, S. Guo, J. Li, E. Wang, and S. Dong, Talanta, 84, 60–64 (2011).
L. Yang, D. Huo, C. Hou, K. He, F. Lv, H. Fa, and X. Luo, Process Biochem., 45, 1664–1671 (2010).
Z. Zheng, X. Li, Z. Dai, S. Liu, and Z. Tang, J. Mater. Chem., 21, 16955–16962 (2011).
A. M. Ashafi, J. Dordevic, V. Guzsvany, T. Trtic-Petrovic, and K. Vytras, XXXII Moderni Electrochemicke Metody, 10–13 (2012).
M. Wang, R. Feng, J. Shen, H. Chen, and Z. Zeng, Bull. Korean Chem. Soc., 33, 2224–2228 (2012).
Y. J. Wu, W. Zhu, and Y. Y. Cheng, Chin. J. Anal. Chem., 34, 235–238 (2006).
C.-J. Hou, K. He, L.-M. Yang, D.-Q. Huo, M. Yang, S. Huang, L. Zhang, and C.-H. Shen, World J. Microbiol. Biotechnol., 28, 541–548 (2012).
H. A. Azab, A. Duerkop, E. M. Saad, F. K. Awad, R. M. Abd El Aal, and R. M. Kamel, Spectrochim. Acta A: Mol. Biomol. Spectrosc., 97, 915–922 (2012).
Y. J. Hu, Y. Liu, R. M. Zhao, J. X. Dong, and S. S. Qu, J. Photochem. Photobiol. A: Chemistry, 179, 324–329 (2006).
L. Yang, D. Huo, C. Hou, M. Yang, H. Fa, and X. Luo, Spectrochim. Acta A: Mol. Biomol. Spectrosc., 78, 1349–1355 (2011).
J. Kang, Y. Liu, M. X. **e, S. Li, M. Jiang, and Y. D. Wang, Biochim. Biophys. Acta: General Subjects, 1674, 205–214 (2004).
J. Gomis, A. Arques, A. M. Amat, M. L. Marin, and M. A. Miranda, Appl. Catal. B: Environ., 123, 208–213 (2012).
Author information
Authors and Affiliations
Additional information
Abstract of article is published in Zhurnal Prikladnoi Spektroskopii, Vol. 85, No. 3, p. 514, May–June, 2018.
Rights and permissions
About this article
Cite this article
Dong, L., Ren, Y., Li, J. et al. Detection of Carbendazim Residues in Aqueous Samples by Fluorescent Quenching of Plant Esterase. J Appl Spectrosc 85, 535–542 (2018). https://doi.org/10.1007/s10812-018-0684-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-018-0684-7