Abstract
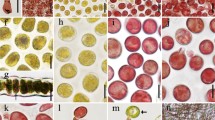
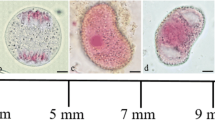
Cytological observations were systematically employed for detecting cellular morphogenesis and chromosomal behavior of apogamous development of new sporophytes from individually cultured male gametophytes of Pyropia haitanensis. The results showed that the apical parts of premature male gametophytes initially underwent obvious apogamy, which primarily appeared in the functionally specialized spermatangial mother cells (SSMCs) that would not develop into highly differentiated spermatangia but change their developmental fate and become brownish red in color. These brownish red spermatangial mother cells (SMCs) were released accompanying the spermatia dissociated from mature spermatangia and then rarely formed circular carpospore-like cells (CLCs) and carpospore-like germlings (CLGs) that eventually developed into normal apogamous sporophytic conchocelis. Chromosome counts indicated that spermatangia and spermatia were haploid (n = 5), whereas SSMCs, CLCs, and CLGs were diploid (2n = 10). Furthermore, filamentous conchocelis and conchosporangial branch cells of apogamous sporophytes were diploid and no haploid, aneuploid, and polyploid were observed, suggesting that spontaneous chromosome doubling occurred in the process of apogamy and initiated rarely SSMCs rather than released spermatia. Moreover, pit connections were observed in the whole development of apogamous sporophytes like in heterozygous sporophytes. Mitotic chromosomal behaviors appeared during the first two divisions of conchospores that normally grew into haploid tetrads and developed into unisexual male gametophytes with the characteristics of apogamy in the aging cultures.





Similar content being viewed by others
Abbreviations
- SSMCs:
-
Specialized spermatangial mother cells
- SCs:
-
Spermatangial cells
- CLCs:
-
Carpospore-like cells
- CLGs:
-
Carpospore-like germlings
- SMCs:
-
Spermatangial mother cells
References
Ar Gall E, Chiang YM, Kloareg B (1993) Isolation and regeneration of protoplasts from Porphyra dentata and Porphyra crispata. Eur J Phycol 28:277–283
Belcher BJH (1960) Culture studies of Bangia atropurpurea (Roth) Ag. New Phytol 59:367–373
Cole KM, Hymes BJ, Sheath RG (1983) Karyotypes and reproductive seasonality of the genus Bangia in British Columbia, Canada. J Phycol 19:136–145
Dai JX, Han BQ, Liu T, Ou YL, Zhuang Y (2000) Properities of monogenetic thalli from male gametophytes of Laminaria japonica Aresch. Mar Sci Bull 19:20–24
Dai JX, Ou YL, Cui JJ, Gong QL, Wang H, Wang ML, Xu JE (1997) Study on the development of male gametophytes of Laminaria japonica Aresch. J Ocean Univ Qingdao 27:41–44
Hawkes MW (1978) Sexual reproduction in Porphyra gardneri (Smith et Hollenberg) Hawkes (Bangiales, Rhodophyta). Phycologia 17:329–353
Jiang Y, Yan XH, Liu CJ (2010) Selection and characterization of a fast growing strain of Porphyra haitanensis (Bangiales, Rhodophyta). J Fish China 34:1363–1370
Kornmann P (1994) Life histories of monostromatic Porphyra species as a basis for taxonomy and classification. Eur J Phycol 29:69–71
Lee RE (1971) The pit connections of some lower red algae: ultrastructure and phylogenetic significance. Br Phycol J 6:29–38
Mitman GG, van der Meer JP (1994) Meiosis blade development, and sex determination in Porphyra purpurea (Rhodophyta). J Phycol 30:147–159
Müller DG, Murúa P, Westermeier R (2019) Reproductive strategies of Lessonia berteroana (Laminariales, Phaeophyceae) gametophytes from Chile: apogamy, parthenogenesis and cross-fertility with L. spicata. J Appl Phycol 31:1475–1481
Nakahara H, Nakamura Y (1973) Parthenogenesis, apogamy and apospory in Alaria crassifolia (Laminariales). Mar Biol 18:327–332
Nelson WA, Brodie J, Guiry MD (1999) Terminology used to describe reproduction and life history stages in the genus Porphyra (Bangiales, Rhodophyta). J Appl Phycol 11:407–410
Notoya M, Iijima N (2003) Life history and sexuality of archeospore and apogamy of Bangia atropurpurea (Roth) Lyngbye (Bangiales, Rhodophyta) from Fukaura and Enoshima. Fish Sci 69:799–805
Pueschel CM, Cole KM (1982) Rhodophycean pit plugs: an ultrastructural survey with taxonomic implications. Am J Bot 69:703–720
Saito A, Mizuta H, Yasui H, Saga N (2008) Artificial production of regenerable free cells in the gametophyte of Porphyra pseudolinearis (Bangiales, Rhodophyceae). Aquaculture 281:138–144
Shin JA, Miura A (1990) Estimation of the degree of self-fertilization in Porphyra yezoensis (Bangiales, Rhodophyta). Hydrobiologia 204:397–400
Sugimoto-Shirasu K, Roberts K (2003) ‘Big it up’: endoreduplication and cell–size control in plants. Curr Opin Plant Biol 6:544–553
Takahashi M, Mikami K (2017) Oxidative stress promotes asexual reproduction and apogamy in the red seaweed Pyropia yezoensis. Front Plant Sci 8:62
Tang XR, Jiang HX, Fei XG, Yarish C (2004) New life cycles of Porphyra katadae var. hemiphylla in culture. J Appl Phycol 16:505–511
Tseng CK, Sun AS (1989) Studies on the alternation of the nuclear phases and chromosome numbers in the life history of some species of Porphyra from China. Bot Mar 32:1–8
van der Meer JP (1990) Genetics. In: Sheath RG, Cole KM (eds) Biology of the Red Algae. Cambridge University Press, Cambridge, pp 103–121
Waaland JR, Dickson LG, Watson BA (1990) Protoplast isolation and regeneration in the marine red alga Porphyra nereocystis. Planta 181:522–528
Wang J, Dai JX (2008) Study on the development of unisexual thalloid cells of Porphyra haitanensis. J Ocean Univ China 38:419–423
Wang SJ, Zhang XP, Xu ZD, Sun YL (1986) A study on the cultivation of the vegetative cells and protoplasts of Porphyra haitanensis. Oceanol Limnol Sin 17:217–221
Wittmann W (1965) Acto–iron–heamatoxylin–chloral hydrate for chromosome staining. Stain Technol 40:161–164
**e CT, Chen CS, Xu Y, Ji DH (2010) Construction of a genetic linkage map for Porphyra haitanensis (Bangiales, Rhodophyta) based on sequence–related amplified polymorphism and simple sequence repeat markers. J Phycol 46:780–787
Xu Y, **e CT, Chen CS, Ji DH, Gao YH (2012) Genetic analyses of six quantitative traits of a doubled haploid population of Porphyra haitanensis Chang et Zheng (Bangiales, Rhodophyta). J Appl Phycol 24:89–96
Yan XH, He LH, Huang J, Song WL, Ma P, Aruga Y (2008) Cytological studies on Porphyra haitanensis Chang et Zheng (Bangiales, Rhodophyta). J Fish China 32:131–137
Yan XH, He LH, Aruga Y (2007a) Karyological observation on the occurrence of meiosis in the life cycle of Porphyra haitanensis Chang et Zheng (Bangiales, Rhodophyta). Bot Mar 50:257–263
Yan XH, Li L, Chen JH, Aruga Y (2007b) Parthenogenesis and isolation of genetic pure strains in Porphyra haitanensis (Bangiales, Rhodophyta). High Technol Lett 17:205–210
Yan XH, Liu XS (2007) Comparison on cell differentiation of male and female blades in Porphyra haitanensis (Bangiales, Rhodophyta). J Fish China 31:184–192
Yan XH, Lv F, Liu CJ, Zheng YF (2010) Selection and characterization of a high-temperature tolerant strain of Porphyra haitanensis Chang et Zheng (Bangiales, Rhodophyta). J Appl Phycol 22:511–516
Zeng QG, Liu BQ, Yang R, Luo QJ, Wang YJ (2004) Morphogeny of conchocelis thalli from single somatic cell clone cultivation of Porphyra haitanensis. J Fish Sci Chin 11:549–552
Zhang Y, Yan XH (2013) Observation on tetrad development and formation of sex phenotype of Pyropia haitanensis blades in natural conditions. J Fish China 37:871–883
Zhang Y, Yan XH, Aruga Y (2013) The sex and sex determination in Pyropia haitanensis (Bangiales, Rhodophyta). PLoS One 8:e73414
Zhong CH, Aruga Y, Yan XH (2019) Morphogenesis and spontaneous chromosome doubling during the parthenogenetic development of haploid female gametophytes in Pyropia haitanensis (Bangiales, Rhodophyta). J Appl Phycol 31:2729–2741
Zhou W, Zhu JY, Shen SD, Lu S, Wang JF, Xu JR, Xu P (2008) Observations on the division characterization of diploid nuclear in Porphyra (Bangiales, Rhodophyta). J Appl Phycol 20:991–999
Acknowledgments
The study was supported in part by the National Key Research and Development Program of China (2018YFD0900606), National Natural Science Foundation of China (31072208), Major Science and Technology Specific Program of Zhejiang Province (2016C02055-6), Science and Technology Planning Project of Jiangsu Province, China (BE2018335), and Open Program of Key Laboratory of Cultivation and High-Value Utilization of Marine Organisms in Fujian Province (2017fjscq02)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhong, C., Yan, X. Haploid spontaneous diploidization during apogamy of male gametophytes in Pyropia haitanensis (Bangiales, Rhodophyta). J Appl Phycol 32, 1395–1403 (2020). https://doi.org/10.1007/s10811-019-01981-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-019-01981-9