Abstract
Purpose
To evaluate the efficacy and safety of adalimumab (ADA, Humira®) for treatment of non-infectious uveitis (NIU) refractory to conventional medications.
Methods
Anti-tumor necrosis factor-α naive patients with NIU unresponsive to conventional immunosuppressive treatment were treated with ADA. Most cases with NIU were related to ocular Behçet syndrome. Adult cases used 80 mg ADA subcutaneously on day 0, 40 mg in the first week, and then 40 mg every 2-week, while this was 20 mg in children. Evaluations were performed pre-treatment and at weeks 2, 8, and 24. The study endpoints were best-corrected visual acuity (BCVA, LogMAR) improvement, anterior chamber (AC) cell grade, vitreous cell and haze grades, decrease in macular thickness and edema, prednisolone dose, immunosuppressive dose, and adverse reactions.
Results
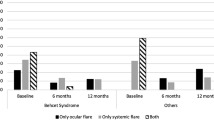
Thirty-eight eyes (19 right, 19 left) of 24 patients (14 female, 10 male) with (ocular Behçet syndrome) OBS (n = 27 eyes/18 patients) and NIU (n = 11 eyes/6 patients) were included. Mean age was 29.0 ± 14.1 years (range, 5–49) and follow-up time was 24 weeks. After ADA, BCVA increased (p < 0.001), and improvements in AC cell grade (p < 0.001), vitreous cell grade (p < 0.001), and vitreal haze grade (p < 0.001) were achieved at the final visit. Mean macular thickness decreased from 243.5 to 235.5 µm (p < 0.001). Such a rapid control of both anterior and posterior uveitis was observed in all eyes as early as the second week without relapses during follow-up. No ocular or systemic complications emerged during treatment.
Conclusions
ADA is effective and well-tolerated in pediatric and adolescent patients with NIU including OBS refractory to traditional medications and demonstrated corticosteroid- and immunosuppressive-sparing effects with no major side effects.





Similar content being viewed by others
References
Evereklioglu C (2005) Current concepts in the etiology and treatment of Behçet disease. Surv Ophthalmol 50:297–350
Evereklioglu C (2004) Managing the symptoms of Behçet’s disease. Expert Opin Pharmacother 5:317–328
Evereklioglu C, Er H, Türköz Y, Çekmen M (2002) Serum levels of TNF-α, sIL-2R, IL-6, and IL-8 are increased and associated with elevated lipid peroxidation in patients with Behçet’s disease. Mediat Inflamm 11:87–93
Evereklioglu C, Borlu M (2008) Sustained remission after infliximab in a child with systemic vasculitis refractory to conventional immunosuppressive therapy including interferon-α. Br J Ophthalmol 92:1034
Evereklioglu C (2011) Ocular Behçet disease: current therapeutic approaches. Curr Opin Ophthalmol 22:508–516
Jabs DA, Nussenblatt RB, Rosenbaum JT (2005) Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. results of the first international workshop. Am J Ophthalmol 140:509–516
Nussenblatt RB, Palestine AG, Chan CC, Roberge F (1985) Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology 924:467–471
Evereklioglu C (2023) Twenty years of quiescence after nonstop Remicade® (Infliximab) infusions in a child with ocular Behçet disease presenting as hypopyon-anterior uveitis refractory to immunosuppressants. Case Rep Ophthalmol 14:75–82
Atienza-Mateo B, Martín-Varillas JL, Calvo-Río V, Demetrio-Pablo R, Beltrán E, Sánchez-Bursón J et al (2019) Comparative study of infliximab versus adalimumab in refractory uveitis due to Behçet’s disease: national multicenter study of 177 cases. Arthritis Rheumatol 71:2081–2089
Díaz-Llopis M, Salom D, Garcia-de-Vicuña C, Cordero-Coma M, Ortega G, Ortego N et al (2012) Treatment of refractory uveitis with adalimumab: a prospective multicenter study of 131 patients. Ophthalmology 119:1575–1581
Calvo-Río V, Blanco R, Beltrán E, Sánchez-Bursón J, Mesquida M, Adán A et al (2014) Anti-TNF-α therapy in patients with refractory uveitis due to Behçet’s disease: a 1-year follow-up study of 124 patients. Rheumatology (Oxford) 53:2223–2231
Jaffe GJ, Dick AD, Brézin AP, Quan Dong Nguyen QD, Thorne JE, Kestelyn P et al (2016) Adalimumab in patients with active noninfectious uveitis. N Engl J Med 375:932–943
Lee JT, Yates WB, Rogers S, Wakefield D, McCluskey P, Lim LL (2018) Adalimumab for the treatment of refractory active and inactive non-infectious uveitis. Br J Ophthalmol 102:1672–1678
Quartier P, Baptiste A, Despert V, Allain-Launay E, Koné-Paut I, Belot A (2018) ADJUVITE Study Group. ADJUVITE: a double-blind, randomised, placebo-controlled trial of adalimumab in early onset, chronic, juvenile idiopathic arthritis-associated anterior uveitis. Ann Rheum Dis 77:1003–1011
Fabiani C, Vitale A, Emmi G, Vannozzi L, Lopalco G, Guerriero S (2017) Efficacy and safety of adalimumab in Behçet’s disease-related uveitis: a multicenter retrospective observational study. Clin Rheumatol 36:183–189
Martín-Varillas JL, Calvo-Río V, Beltrán E, Sánchez-Bursón J, Mesquida M, Adán A (2018) Successful optimization of adalimumab therapy in refractory uveitis due to Behçet’s disease. Ophthalmology 125:1444–1451
Ho M, Chen LJ, Sin HPY, Lu LPL, Brelen M, Ho ACH (2019) Experience of using adalimumab in treating sight-threatening paediatric or adolescent Behcet’s disease-related uveitis. J Ophthalmic Inflamm Infect 9:14
Silvestri E, Bitossi A, Bettiol A, Emmi G, Urban ML, Mattioli I (2020) Adalimumab effectively controls both anterior and posterior noninfectious uveitis associated with systemic inflammatory diseases: focus on Behçet’s syndrome. Inflammopharmacology 28:711–718
Yang S, Huang Z, Liu X, Li H, **e L, Chen X (2021) Comparative study of adalimumab versus conventional therapy in sight-threatening refractory Behçet’s uveitis with vasculitis. Int Immunopharmacol 93:107430
Funding
The authors declare that no funds, grands, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. CE contributed to conceptualization; HKS helped in methodology; DGS and HA performed formal analysis and investigation; CE and OAP contributed to writing—original draft preparation; HŞ and FH helped in writing—review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interest to disclose.
Consent to participate
Informed consent was obtained from all individual participants, and written informed consent was obtained from the parents of pediatric cases in this study.
Consent for publication
This submission has not been published anywhere previously and it is not simultaneously being considered for any other publication.
Ethics approval
The study was reviewed and approved by the department’s academic board and the ethics committee of Erciyes University, Kayseri, Türkiye (No: 2020/76).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Evereklioglu, C., Sonmez, H.K., Sevim, D.G. et al. Adalimumab rapidly controls both anterior and posterior inflammation in patients with ocular Behçet syndrome and non-infectious uveitis refractory to conventional therapy: a prospective, 6-month follow-up investigation. Int Ophthalmol 43, 4461–4472 (2023). https://doi.org/10.1007/s10792-023-02846-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-023-02846-4