Abstract—
Lung epithelium is constantly exposed to the environment and is critically important for the orchestration of initial responses to infectious organisms, toxins, and allergic stimuli, and maintenance of normal gaseous exchange and pulmonary function. The integrity of lung epithelium, fluid balance, and transport of molecules is dictated by the tight junctions (TJs). The TJs are formed between adjacent cells. We have focused on the topic of the TJ structure and function in lung epithelial cells. This review includes a summary of the last twenty years of literature reports published on the disrupted TJs and epithelial barrier in various lung conditions and expression and regulation of specific TJ proteins against pathogenic stimuli. We discuss the molecular signaling and crosstalk among signaling pathways that control the TJ structure and function. The Toll-like receptor-4 (TLR4) recognizes the pathogen- and damage-associated molecular patterns released during lung injury and inflammation and coordinates cellular responses. The molecular aspects of TLR4 signaling in the context of TJs or the epithelial barrier are not fully known. We describe the current knowledge and possible networking of the TLR4-signaling with cellular and molecular mechanisms of TJs, lung epithelial barrier function, and resistance to treatment strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Maintenance of cellular and tissue architecture and function is critical for the normal physiology of body organs and specifically the mucosal gastrointestinal and pulmonary systems, which are constantly exposed to foreign particles, pathogens, toxicants, and environmental stimuli. While recognition and elimination of harmful stimuli are coordinated by nonspecific mechanisms elicited by anatomic structures, such as cilia, immune and non-immune endothelial and epithelial cells can respond in a unique manner against pathologic ligand or stimuli. For simplicity, we have focused on addressing the epithelial layer integrity and function in normal and injured lung during infections and inflammatory conditions. In the respiratory system, the bronchi and bronchioles are mainly equipped with ciliary epithelial cells, mucus-secreting goblet cells, and Clara cells. The alveolar region consists of highly specialized type I and type II epithelial cells. The epithelial cells are connected via tight junction (TJ) proteins on their apical surface at the air–liquid interface. Here we describe the epithelial integrity and coordination of lung function in the context of molecular interplay under normal and injury conditions.
AIRWAY AND ALVEOLAR EPITHELIUM
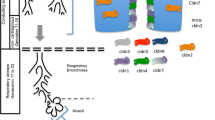
The human airways can be divided into conducting and respiratory airways. The airway epithelium is pseudostratified in the large airways, becoming columnar and cuboidal in the small airways. The major cells in conducting airways are ciliated, pre-ciliated, basal, goblet, secreted, and indeterminate undifferentiated types. These epithelial cell populations vary at different levels of airways; the numbers of secretory cells increase as the conducting airways start to branch from large to small airways [1]. The alveolar sacs are at the extreme end of respiratory airways. The alveolar epithelium is mainly composed of type I and type II epithelial cells. The type I alveolar epithelial cells are more flattened cells, cover 93% of the alveolar surface, and provide a large surface for gaseous exchange [2]. The type II epithelial cells are specialized cuboidal cells that synthesize surfactant, which is packaged into lamellar body structures and secreted in the alveoli as tubular myelin. The surfactant is a mixture of proteins and lipids and plays an important role in lung function and host defense against invading pathogens and noxious stimulants. The type II epithelial cells serve as progenitor cells for both type I and type II alveolar epithelial cells [2]. An article published by Crystal et al. provides a comprehensive review of airway epithelial cells, stem/progenitor cells, and interaction among cell types [3].
The formation of adhesive contacts between cells is essential for the function of many tissues. This is particularly true for epithelial cells in the lung, which adhere tightly to one another to form an epithelial sheet. This epithelial lining acts as a barrier between airways or alveolar sacs and the underlying interstitial and endothelial layers. The junctional complexes between the neighboring epithelial cells consist of TJs just underneath the apical surface and adherens junctions (AJs) below TJs, at the basolateral sides of the cells [4].
The TJs form the functional and structural boundary that separates apical and basolateral compartments [5], and facilitate intercellular adhesion just underneath the apical surface; control the passage of ions, water, and other molecules; and help maintain the cell polarity [6]. The TJs of the pulmonary epithelium provide the structural basis for the air-blood carrier that prevents invasion of inhaled pathogens and prevents non-selective leakage of fluid into the air spaces [7]. Various studies have revealed the architecture of TJs, which consist of transmembrane and peripheral membrane proteins (Table 1). Transmembrane TJ proteins on airway epithelial cells consist of three main families of proteins: the tight junction associated Marvel proteins (TAMPs), which share myelin and lymphocyte protein (MAL) and related proteins for vesicle trafficking and membrane link (MARVEL) domain, claudins, and Ig superfamily proteins (e.g., junctional adhesion molecules [JAM] and coxsackievirus B adenovirus receptor [CAR]) [8]. Peripheral TJ proteins include F-actin, F-actin-binding scaffold proteins, non-F-actin-binding scaffold proteins, and cell polarity and signaling molecules. The zonula occludens (ZO) proteins are the major peripheral membrane proteins. Transmembrane proteins of the TJs bind to peripheral membrane TJ proteins via their intracellular domains and organize the cellular signal transduction and cellular responses. The ZO proteins as part of TJs connect with the AJs and cytoskeleton composed of actin [9].
While we have mainly focused on TJs, AJs are positioned immediately below the TJs in the cells. Both TJ and AJ proteins provide polarity and help maintain intercellular adhesion. The AJ proteins consist of two basic units: the nectin–afadin complex and the classical cadherin-catenin complex. Nectin is an immunoglobulin-like intercellular adhesion molecule, and afadin is a nectin- and actin-filament binding protein that connects nectin to the actin cytoskeleton. The nectin adhesion system organizes E-cadherin-based AJs and claudin-based TJs in epithelial cells [10]. E-cadherin contains an extracellular domain that forms homotypic, calcium-dependent adhesions between epithelial cells. It is associated with the actin cytoskeleton through catenins. The actin microtubule cytoskeleton, a dynamic structure, maintains the cell shape and enables cilia motion, vesicle transport, and cell proliferation [11]. At the basolateral side of E-cadherin-mediated cell–cell contacts, the desmosomes, consisting of nonclassical cadherins, are formed, providing mechanical strength to the tissues. Gap junctions, or membrane channels, are formed between the neighboring cells through connexins, which allow the direct passage of ions, secondary messengers, and metabolites [12]. The AJs are critical for cell–cell contact at the basolateral surface, and the TJ proteins play an important role in relaying the molecular signal to its intracellular molecular partners from the periphery of the cells.
GENETIC MOUSE MODELS OF TJ PROTEINS
Investigations in genetic mouse models have highlighted the importance of different TJ proteins in maintaining epithelial polarity and barrier function. Knockdown of individual claudins in mice revealed mild lung phenotypes, suggesting that the other proteins in the lung can compensate for the loss of claudin function to some extent; the claudin-4 and claudin-18 knockout mice demonstrated increased solute permeability and alveolar fluid clearance [13,14,15,16,17]. JAM-A-deficient mice demonstrated increased susceptibility to pulmonary edema against lipopolysaccharide (LPS) challenge, which correlates with a transient disruption of claudin-18, ZO-1, and ZO-2 localizations to TJs in the lung and a delay in upregulation of claudin-4 [18]. A mouse model with conditional loss of E-cadherin in lung epithelial cells showed normal lung development at the time of birth, but progressive epithelial damage in adulthood, as evidenced by airway epithelial denudation, decreased ZO-1 expression, loss of ciliated cells, and enlarged alveolar spaces [19]. An afadin knockdown attenuates the interaction between ZO-1 and p120-catenin proteins [20]. Targeted disruption of either ZO-1 or ZO-2 in mice results in embryonic death that correlates with disruption of the paracellular barrier and the structure of cell junctions. The ZO-1 gene-deletion is associated with a defective organization of notochord, neural tube, and allantois and is embryonically lethal in mice at E10.5 to E11.5 [21]. Similarly, ZO-2-deleted mice do not survive beyond the embryo stage due to compromised TJ structure and function [22]. In contrast to the ZO-1 and ZO-2 null phenotypes, mice and cell systems with the ZO-3 gene deletion express no abnormalities. These observations suggest the dispensable function of ZO-3 [23]. Occludin knockout mice are viable with no morphological effect on TJs or intestinal barrier function. However, histological abnormalities are observed in different tissues: chronic inflammation and hyperplasia of gastric epithelium, testicular atrophy, salivary gland dysfunction, thinning of compact bone, and brain calcifications. The occludin deficiency in genetic mice does not affect the expression or localization of claudin-3 or claudin-1 in the intestinal epithelial cells [24, 25]. The phenotype of occludin-deficient mice has been linked by relocalization of tricellulin from tricellular to bicellular junctions [26, 27]. The double knockout of the occludin and tricellulin genes reduces the strand-to-strand junction points of the TJ strands, whereas loss of either occludin or tricellulin has no drastic effects. The MarvelD3 could also have a redundant role with tricellulin and occludin. Claudin-18 deficiency results in alveolar barrier dysfunction and impaired alveologenesis [13, 15]. Impaired TJ structure and/or function have also been reported in mice deficient in lymphoblastic leukemia-derived sequence (Lyl)-1, Crumbs (Crb3), and sirtuins (SIRTs) [28,29,30].
TJ PROTEINS AND MEMBRANE PERMEABILITY IN LUNG INJURY
Lung injuries manifested as non-cardiogenic pulmonary edema, respiratory distress, and hypoxemia result from various types of stimuli that directly or indirectly injure the lung. Lung injuries associated with acute respiratory distress syndrome (ARDS) are characterized by exposure to a known risk factor or worsening of the respiratory symptoms within 1 week, bilateral opacities on chest imaging (radiological assessment), edema, and oxygenation status indicated by the ratio of arterial oxygen partial pressure (PaO2 in mmHg) to fractional inspired oxygen (FiO2 expressed as a fraction; physiological criteria) [31]. A pro-inflammatory increase in vascular permeability and neutrophil infiltration, lung edema, and widespread damage to lung cells, biochemical components, structures, and alveolar barrier are hallmarks of lung injury [31, 32].
The airway epithelium acts as a physical barrier to prevent the entry of potential pathogens or noxious environmental stimulants across the airway mucosa [33]. Damage or dysfunction of the TJs results the deterioration of the epithelial cell function, increased permeability, and lung edema. Alteration of the TJs facilitates the passage of infectious agents, exogenous toxins and endogenous products into the systemic circulation, contributing to ARDS and multi-organ failure [34]. For the purposes of this review, we performed a PubMed search (2000–2021) using a combination of keywords (lung injury and tight junction; lung injury and membrane permeability; lung inflammation and membrane permeability; ARDS and tight junction; ARDS and membrane permeability). The association of altered expression and localization of TJ proteins with various lung conditions is summarized in the Supplemental Table.
TJ ASSEMBLY AND FUNCTION
The TJs are heavily cross-linked structures and are dynamic in nature, presenting challenges for detailed investigations at molecular and cellular levels [35]. The pharmacological modulators, genetically modified cells, and animal models have facilitated studies on the TJs. The transmembrane TJ proteins (e.g., claudins and occludin) form strands between neighboring cells. These proteins connect with the actin-myosin cytoskeleton through their interaction with scaffolding protein, mainly ZOs [36]. Despite a great understanding of many TJ proteins, the components of the selectively permeable barrier are not clearly known. Rapid freezing of the newly formed TJ barrier indicated cylinder-like structures, which were interpreted as inverted phospholipid micelles [37]. Additional pieces of evidence for barrier loss after cholesterol depletion [38] and interaction of occludin with lipid raft component caveolin-1 [39,40,41] suggest the role of membrane lipids in TJ formation.
TJ assembly and function are known to be controlled by the Ras homolog (Rho)GTPase. The activation of RhoGTPase is regulated by GDP to GTP nucleotide exchange factors and by GTP hydrolysis. The RhoGTPase stimulates specific effectors known as Rho kinases (ROCKs). The ROCKs phosphorylate and activate myosin light chain II (MLC) [42, 43] via two mechanisms, direct phosphorylation of MLC and indirect phosphorylation of the regulatory subunit of MLC phosphatase (MYPT1), which suppress MLC phosphatase activity [44]. The ATP-dependent ratcheting of actin and myosin is catalyzed by the calcium calmodulin-dependent non-muscle MLC kinase (MLCK) [45]. Several TJ proteins directly interact with actin and myosin, providing stability for the TJ complex.
The RhoGTPase/ROCK signaling participates in both assembly and disruption of TJs [46, 47]. The disruption or opening of TJs as a result of infections, inflammation, and toxic stimuli involves the phosphorylation of MLC2 and contraction of the actomyosin ring. Additional signaling molecules include mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinases (ERK1/2), mechanistic target of rapamycin (mTOR), caveolin-1, intracellular Ca2+-dependent mechanism, G protein, protein kinase C, phosphoinositol 3 kinase (PI3K), protein phosphatases, phosphorylation-related regulation, and small GTPases, depending on the tissue microenvironment [48,49,50,51,52,53].
TJ DISRUPTION DURING LUNG INJURY AGAINST INFECTIOUS AND INFLAMMATORY STIMULI
Normally, the lower airways are sterile, free from bacteria or inflammatory cells, and well protected by several layers of defenses, including antimicrobial peptides and mucins. Significant changes have been identified in TJ proteins in lung cell systems, animal models, and human patients suffering from lung injury, inflammation, and ARDS. Alterations in TJs are often characterized by reduced TJ strand formation, strand breaks, and altered TJ protein expression and distribution. Pulmonary insults with environmental stimulants and pathogens initiate the injury at the alveolar side of the alveolar-capillary barrier. Many studies have focused on the capillary endothelial barrier; little has been investigated about the barrier function of the alveolar epithelium. Mechanical stretch [54]; viruses, such as SARS-CoV-2, and bacterial pathogens [55,56,57,58]; pathogen-associated molecular patterns (PAMPs, e.g., LPS) [18]; inflammatory cytokines, such as interleukin (IL)-4, IL-13, tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ); and reactive oxygen species [59] are known to disrupt TJ assembly at the epithelial side of the barrier. We describe the current understanding on the cellular and molecular mechanisms of TJ disruption mainly at the level of alveolar epithelial cells during lung injury and inflammation. Type I and type II alveolar epithelial cells differ in their morphology and function, including the expression of the claudins [12, 60].
The mechanical stretch mimicking the ventilation-induced lung injury increases the phosphorylation of protein kinase RNA-like endoplasmic reticulum kinase (p-PERK) and integrated stress response (ISR) marked by eukaryotic initiation factor-2alpha (p-ELF2alpha), activating transcription factor-4 (ATF-4), and CCAAT/enhancer-binding protein homologous protein (CHOP), eventually resulting in permeability changes in primary alveolar type 1-like epithelial cells [61]. The cyclic stretches (20%) activated cSrc, induces degradation of E-cadherin, p120, and occludin in the mouse lung epithelial cell system [62]. The alveolar epithelial cells stretch leads to an increase in protein kinase B or Akt and LIM kinase and decrease in cofilin phosphorylation, suggesting involvement of the Ras-related C3 botulinum toxin substrate (Rac1)/Akt pathway [63].
The TJs and lung epithelial barrier are directly affected by pathogens or PAMPs: tyrosine kinase BceF and the phosphotyrosine phosphatase BceD Burkholderia contaminans [64], Pseudomonas aeruginosa [65,66,67], Stenotrophomonas maltophilia protease, Bacillus anthracis [68], and viruses (respiratory syncytial virus [RSV], rhinovirus, coxsackievirus, adenovirus, influenza, SARS-CoV-2, papillomavirus, and vaccinia virus) [69,70,71]. Pore-forming toxins released from bacteria induce necroptosis in lung epithelial cells as a result of ion dysregulation arising from membrane permeabilization [72].
The cytokines deregulate epithelial sodium channel (ENaC) activity, which results in fluid accumulation and alveolar flood, loss of normal gas exchange, and hypoxemia [73]. Among various cytokines, TNF-α and IL-1β increase pulmonary epithelial permeability [74,75,76]. TNF-α has been shown to alter the expression and distribution of TJ proteins (ZO-1, claudin-2, claudin-4, and claudin-5) and β-catenin [77], remodel actin for the formation of contractile stress fibers [78], and disrupt epithelial barrier function [79]. The TNF-α induces ceramide levels in lung epithelial cells, which leads to generation of sphingosine. Sphingosine is rapidly phosphorylated to sphingosine-1-phosphate (S1P), which activates RhoGTPase and ROCK and helps control the barrier integrity in endothelial cells [80]. An accumulation of ceramide decreases the barrier function of alveolar type II epithelial cells [81]. The ceramide and sphingomyelin metabolites are also known to suppress surfactant phosphatidylcholine synthesis in lung epithelial cells via protein kinase C (PKC)-α, p38 MAPK, cytosolic phospholipase A2 (cPLA2), and 5-lipoxygenase [82, 83]. In human airway epithelial cells, inhibition of the src-kinase attenuates TNF-α-induced TJ disruption and partly restores the TJ [84]. The inflammatory changes and lung TJ function are subdued by treatment with Etanercept, a TNF-α soluble receptor antagonist [77, 85]. The IL-1β activates transforming growth factor (TGF)-β through RhoA/αβ6 integrin-dependent mechanisms. Inhibition of the αβ6 integrin and/or TGF-β signaling suppresses the IL-1β-mediated protein permeability across alveolar epithelial cell monolayer [86]. ZO-1 and occludin expression and lung epithelial barrier function are also reduced by IL-4 and IL-13, but are enhanced by IFN-γ [87]. Prostaglandin E2 production is associated with p38 and c-jun N-terminal kinase, cPLA2, cyclooxygenase (COX)-2 mRNA, and disruption of the membrane barrier in human alveolar epithelial cells [88]. Pro-inflammatory thrombin and histamine modulate RhoGTPase-ROCK-MLCK and actomyosin contraction in endothelial cells through PKC (reviewed in [89]).
The damage-associated molecular patterns (DAMPs), such as hyaluronic acid binding protein-2 (HABP2), released during injury negatively regulate the vascular integrity via activation of protease-activated receptor (PAR)/RhoA/ ROCK signaling [90]. The mitochondrial DAMPs, such as fragmented mitochondria, induce MAPK phosphorylation in endothelial cells, which increases polymorphonuclear leukocytes (PMN) adherence and membrane permeability. The endosomal TLR inhibitor chloroquine has been shown to inhibit the permeability changes in endothelial cells [91].
It is now well recognized that TNF-α downregulates epithelial barrier function via activating nuclear factor (NF)-κB. NF-κB can directly modulate the TJ permeability [92]. The activation of NF-κB leads to the release of other inflammatory mediators, such as IL-6 and IL-1β, which have been shown to increase the airway permeability [93, 94].
Two pathways have been proposed for the paracellular movement of molecules. The claudin-containing “pore” controls the movement of ions in a charge- and size-selective manner, and the “leak” pathway allows limited movement of large macromolecules [95, 96]. The cell signaling at TJs is bidirectional, such that signals are transmitted from the cell interior to TJs and vice versa. For example, the claudins form the paracellular cation and water permeable channels for transport of solutes and ions in both directions and affect cell proliferation and migration [97].
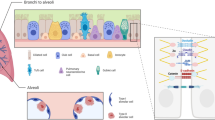
The expression of claudin-4 and claudin-18 is regulated in an opposite manner during lung injury. An increase in claudin-4, but a decrease in claudin-18, is observed during injury (reviewed in [96]). Data show that the PKC activation increases claudin-4 expression in primary rat and human lung epithelial cells, and jun N-terminal kinase (JNK) inhibition blocks this increase in claudin-4. Findings from experimental and human lung studies suggest that claudin-4 supports the repair of epithelial barrier [96]. During the repair phase, the hepatocyte growth factor (HGF) protects the pulmonary endothelial cell cytoskeleton via Rho- and Rac-specific nucleotide exchange factor Tiam 1/Rac-GTPase-mediated pathways [98, 128]. The LPS secreted by Gram-negative bacteria is the most potent ligand for TLR4. A number of other PAMPs, including SARS-CoV-2 viral protein, and DAMPs released during inflammation and injury are also sensed by TLR4. High mobility group box 1 protein (HMGB1) release also induces TLR4 and PKC-mediated internalization of surface TJs in the pulmonary epithelium [129]. The alarmins S100A8/A9 promote lung injury through activation of alveolar epithelial cells in a TLR4-dependent manner [130]. After the recognition of PAMPs and DAMPs, activated TLR4 induces a complex network of intracellular signaling pathways, including association with its intracellular co-receptor molecules (myeloid differentiation primary response 88 [MYD88] and toll-interleukin 1 receptor [TIR] domain containing adaptor protein inducing IFN-β [TRIF]), stimulation of nuclear factor (NF)-κB, activator protein (AP)-1, and interferon-regulatory factor (IRF) transcription factors, PI3K/Akt/mTOR and PKC pathways, and priming of inflammasome for persistent inflammatory response (Fig. 1). LPS stimulates mTOR phosphorylation and decreases microtubule-associated protein-1 light chain 3 beta (MAP1LC3B/LC3B)-II, a marker of autophagy, in mouse lung epithelium and human bronchial epithelial cells. The activation of mTOR is mediated by TLR4 signaling. Specific knockdown of mTOR in epithelial cells in mice attenuates barrier disruption [50]. The co-culture of mesenchymal stem cells reduces inflammation in LPS-stimulated alveolar epithelial cells via modulation of the TLR4-signaling pathway by an enhanced secretion of KGF and angiopoietin [131].
Illustration of activated TLR4 signaling by recognition of pathogen- and damage-associated molecular patterns (PAMPs and DAMPs) present in the extracellular milieu and organization of TJ proteins at the apical surface of epithelial cells. While the expression and activity of TLR4 are increased, the disruption of TJ proteins and actin cytoskeleton leads to compromised barrier function during lung injury. An activated TLR4 forms a transmembrane complex upon binding to its ligand and is internalized in the cell. Hypothetically, the TLR4 complex may associate with TJs (A) or downstream TLR4-signaling and effector molecules (B) and affect the TJ assembly and compromise the epithelial barrier function. The circadian rhythm and yet unknown mechanisms could potentially regulate TLR4-signaling, TJ assembly, and barrier function. Intracellular co-receptors of TLR4-signaling pathway (MYD88, myeloid differentiation primary response 88, TIRAP, toll-interleukin 1 receptor [TIR] domain containing adaptor protein, TRAM, TRIF-related adaptor molecule, TRIF, TIR-domain-containing adaptor protein inducing interferon-β) are identified within the figure.
GENETIC AND PHARMACOLOGICAL MODULATION OF TLR4, PERMEABILITY, TIGHT JUNCTION INTEGRITY, AND LUNG INJURY
Studies using pharmacological modulators and agents, and investigations in genetic mouse models have suggested the role of TLR4 in permeability and lung injury. Significantly lower amounts of total protein were detected in bronchoalveolar lavage fluid (BALF) of the C3H/HeJ mice than in those of C3H/OuJ mice against exposure to ozone [122]. The TLR4 deficiency prevents the LPS-induced pulmonary injury [132]. The involvement of TLR4 in disruption of TJs is further emphasized by the neutralization of DAMPs, like HMGB1 [129], and blockade of TLR4 with synthetic compounds or natural products, such as catalpol [133], piceatannol [134], oxycodone [135], madecassoside [136], Houttuynia cordata polysaccharides [137], Schisandrin [138], Ma-**ng-Shi-Gan-Tang [139], Xuebi**g (XBJ) [140], and Sargassum horneri (Turner) [141]. Saquinavir, an inhibitor of HIV protease, also ameliorates the LPS-induced lung injury and permeability with lowered TLR4, but enhanced VE-cadherin [142]. Common observations relate with an increase in TLR4 expression and permeability and reduced expression or disrupted localization of TJ proteins in these lung injury models, which are reversed by these agents for a protective response. Some chemical components, such as Hippophae rhamnoides polysaccharide and selenium-enriched yeast, regulated the TLR4 and NF-κB and increased the expression of claudin-1, ZO-1, and occludin in intestinal injury models [143, 144]. Although the specificity of the modulation of TLR4 signaling is uncertain in the abovementioned studies with different chemical compounds and agents, the results suggest a link between TLR4 and increased permeability via TJ disruption during lung injury.
TREATMENT WITH CORTICOSTEROIDS
Treatment with corticosteroids is the mainstay for patients with lung injury conditions, including ARDS and asthma, despite inconsistent evidence of their efficacy with different clinical protocols. An improvement in patients’ condition has been reported in some studies. However, clinical data also show limited or no effect on patients’ outcomes, even at high treatment doses [145,146,147,148,149,150,151,152,153,154,155,156].
The anti-inflammatory effects of corticosteroids are recognized by the inhibition of leukocyte traffic and migration at the site of inflammation, perturbed cell functions, and suppression of the production of inflammatory molecules and mediators [157, 158]. Lipophilic synthetic corticosteroids easily cross the plasma membrane and bind to cytosolic glucocorticoid receptor, a member of the steroid hormone receptor family of ligand-inducible transcription factors. Upon binding to the ligand, the glucocorticoid receptor undergoes conformation change and translocates into the nucleus [159]. Interaction with glucocorticoid response elements in the nucleus increases the transcription of anti-inflammatory genes. A suppressed synthesis of pro-inflammatory proteins or transrepression results from competition for nuclear coactivators or direct or indirect interaction with transcription factors. Non-genomic pathways are equally important in mediating the effects of corticosteroids [160]. Treatment with corticosteroids affects the expression of TLR4 and IL-8 [161], but upregulates the expression of claudin-8 and decreases the paracellular permeability in a lung epithelial cell culture model of lung injury [162].
ROLE OF TLR4 IN RESISTANCE TO CORTICOSTEROID TREATMENT
As mentioned above, many patients do not respond to corticosteroid treatment. Several mechanisms may exist for steroid resistance, and these may differ in patients who are non-responsive to corticosteroid treatment [151]. Increased expression of the non-responsive beta isoform of glucocorticoid receptor, activation of p38 MAPK leading to phosphorylation of glucocorticoid receptors and reduced corticosteroid binding affinity within nucleus, excessive activation of the JNK pathway, reduced suppression or no effect of corticosteroid on cytokine release, impaired nuclear localization of glucocorticoid receptors, and defective histone acetylation are some of the plausible reasons for steroid-resistance [163,164,165,166]. One report has also identified that the circadian clock gene disruption corroborates with the loss of efficacy of synthetic dexamethasone [114]. Recent reports suggest that steroid resistance is strongly associated with activation of innate immune responses elicited by TLR2, TLR4, and NLRP3 inflammasomes [167, 168]. As such, the corticosteroids significantly compromise the bactericidal mechanisms in alveolar macrophages and in a mouse model of pneumococcal pneumonia [169]. Treatment with corticosteroids is associated with an increased risk of community-acquired pneumonia in patients with COPD [170, 171]. Patients with corticosteroid-resistant asthma demonstrate airway expansion of specific Gram-negative bacteria, which trigger MYD88-dependent transforming growth factor-β-associated kinase-1 activation, resulting in p38 MAPK phosphorylation, NF-κB activation, and transcription of pro-inflammatory cytokines [172]. Repeated exposure to LPS has been shown to activate PI3K, resulting in decreased levels of Histone deacetylase-2 and NF-E2-related factor 2 (Nrf2) and corticosteroid-insensitive airway inflammation [173]. Furthermore, sensitization with LPS in combination with β-glucan (fungal ligand) synergistically promotes corticosteroid refractory neutrophilic inflammation [174]. A cooperative signaling between interferon-γ and LPS-induced TLR4/MYD88 has also been shown to regulate macrophage-dependent, steroid-resistant inflammation [175, 176]. While the corticosteroids exert anti-inflammatory effects to some extent, the significance of unaltered NF-κB-DNA binding and activation and an increase in expression of the p65 component of NF-κB remains unknown [177].
TLR4, CIRCADIAN RHYTHM, AND DISRUPTION OF TJ PROTEINS IN THE LUNG
After recognition of its ligand, the TLR4 activates a complex network of intracellular signaling, resulting in activation of NF-κB, AP-1, and IRF3 transcription factors, PKCs, MAPKs, PI3K, and inflammatory cytokines and chemokines (Fig. 1). As described above and in Table 2, TLR4 activation and decreased expression and/or localization of TJ proteins are unequivocally noted during lung injury. An increased expression and activity of the aggravating downstream molecules, cytokines and chemokines, PAMPs, and DAMPs are evident in tissues and fluids of animal models and patients with lung injury. However, there is little information regarding whether an activated TLR4 forming a transmembrane complex with its co-receptor molecules can directly interact with TJs or whether the downstream TLR4-signaling molecules can affect the expression, assembly, and localization of TJs in lung epithelial cells. The circadian regulation of TLR4 signaling and organization of TJ proteins remains to be studied in lung cell systems and disease models. A thorough understanding of molecular events, signaling, and networks within different lung compartments and cells can facilitate development of novel therapies for restoration of the lung barrier, function and homeostasis, and control of severe outcomes in patients with lung inflammatory and injury conditions.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
REFERENCES
Mercer, R.R., M.L. Russell, V.L. Roggli, and J.D. Crapo. 1994. Cell number and distribution in human and rat airways. American Journal of Respiratory Cell and Molecular Biology 10 (6): 613–624.
Ward, H.E., and T.E. Nicholas. 1984. Alveolar type I and type II cells. Australian and New Zealand Journal of Medicine 14 (5 Suppl 3): 731–734.
Crystal, R.G., S.H. Randell, J.F. Engelhardt, J. Voynow, and M.E. Sunday. 2008. Airway epithelial cells: Current concepts and challenges. Proceedings of the American Thoracic Society 5 (7): 772–777.
Rezaee, F., and S.N. Georas. 2014. Breaking barriers. New insights into airway epithelial barrier function in health and disease. American Journal of Respiratory Cell and Molecular Biology 50(5): 857–869.
Flynn, A.N., O.A. Itani, T.O. Moninger, and M.J. Welsh. 2009. Acute regulation of tight junction ion selectivity in human airway epithelia. Proceedings of the National Academy of Sciences of the United States of America 106 (9): 3591–3596.
Feldman, G.J., J.M. Mullin, and M.P. Ryan. 2005. Occludin: Structure, function and regulation. Advanced Drug Delivery Reviews 57 (6): 883–917.
Tobioka, H., Y. Tokunaga, H. Isomura, Y. Kokai, J. Yamaguchi, and N. Sawada. 2004. Expression of occludin, a tight-junction-associated protein, in human lung carcinomas. Virchows Archiv 445 (5): 472–476.
Kawabe, H., H. Nakanishi, M. Asada, A. Fukuhara, K. Morimoto, M. Takeuchi, and Y. Takai. 2001. Pilt, a novel peripheral membrane protein at tight junctions in epithelial cells. Journal of Biological Chemistry 276 (51): 48350–48355.
Fanning, A.S., C.M. Van Itallie, and J.M. Anderson. 2012. Zonula occludens-1 and -2 regulate apical cell structure and the zonula adherens cytoskeleton in polarized epithelia. Molecular Biology of the Cell 23 (4): 577–590.
Takai, Y., and H. Nakanishi. 2003. Nectin and afadin: Novel organizers of intercellular junctions. Journal of Cell Science 116 (Pt 1): 17–27.
Nawijn, M.C., T.L. Hackett, D.S. Postma, A.J. van Oosterhout, and I.H. Hei**k. 2011. E-cadherin: Gatekeeper of airway mucosa and allergic sensitization. Trends in Immunology 32 (6): 248–255.
Koval, M. 2002. Sharing signals: Connecting lung epithelial cells with gap junction channels. American Journal of Physiology. Lung Cellular and Molecular Physiology 283 (5): L875-893.
Li, G., P. Flodby, J. Luo, H. Kage, A. Sipos, D. Gao, Y. Ji, L.L. Beard, C.N. Marconett, L. DeMaio, Y.H. Kim, K.J. Kim, I.A. Laird-Offringa, P. Minoo, J.M. Liebler, B. Zhou, E.D. Crandall, and Z. Borok. 2014. Knockout mice reveal key roles for claudin 18 in alveolar barrier properties and fluid homeostasis. American Journal of Respiratory Cell and Molecular Biology 51 (2): 210–222.
Kage, H., P. Flodby, D. Gao, Y.H. Kim, C.N. Marconett, L. DeMaio, K.J. Kim, E.D. Crandall, and Z. Borok. 2014. Claudin 4 knockout mice: Normal physiological phenotype with increased susceptibility to lung injury. American Journal of Physiology. Lung Cellular and Molecular Physiology 307 (7): L524-536.
LaFemina, M.J., K.M. Sutherland, T. Bentley, L.W. Gonzales, L. Allen, C.J. Chapin, D. Rokkam, K.A. Sweerus, L.G. Dobbs, P.L. Ballard, and J.A. Frank. 2014. Claudin-18 deficiency results in alveolar barrier dysfunction and impaired alveologenesis in mice. American Journal of Respiratory Cell and Molecular Biology 51 (4): 550–558.
Tokumasu, R., K. Yamaga, Y. Yamazaki, H. Murota, K. Suzuki, A. Tamura, K. Bando, Y. Furuta, I. Katayama, and S. Tsukita. 2016. Dose-dependent role of claudin-1 in vivo in orchestrating features of atopic dermatitis. Proceedings of the National Academy of Sciences of the United States of America 113 (28): E4061–4068.
Ding, L., Z. Lu, O. Foreman, R. Tatum, Q. Lu, R. Renegar, J. Cao, and Y.H. Chen. 2012. Inflammation and disruption of the mucosal architecture in claudin-7-deficient mice. Gastroenterology 142 (2): 305–315.
Mitchell, L.A., C. Ward, M. Kwon, P.O. Mitchell, D.A. Quintero, A. Nusrat, C.A. Parkos, and M. Koval. 2015. Junctional adhesion molecule A promotes epithelial tight junction assembly to augment lung barrier function. American Journal of Pathology 185 (2): 372–386.
Post, S., I.H. Hei**k, L. Hesse, H.K. Koo, F. Shaheen, M. Fouadi, V.N.S. Kuchibhotla, B.N. Lambrecht, A.J.M. Van Oosterhout, T.L. Hackett, and M.C. Nawijn. 2018. Characterization of a lung epithelium specific E-cadherin knock-out model: Implications for obstructive lung pathology. Scientific Reports 8 (1): 13275.
Birukova, A.A., P. Fu, T. Wu, O. Dubrovskyi, N. Sarich, V. Poroyko, and K.G. Birukov. 2012. Afadin controls p120-catenin-ZO-1 interactions leading to endothelial barrier enhancement by oxidized phospholipids. Journal of Cellular Physiology 227 (5): 1883–1890.
Katsuno, T., K. Umeda, T. Matsui, M. Hata, A. Tamura, M. Itoh, K. Takeuchi, T. Fujimori, Y. Nabeshima, T. Noda, S. Tsukita, and S. Tsukita. 2008. Deficiency of zonula occludens-1 causes embryonic lethal phenotype associated with defected yolk sac angiogenesis and apoptosis of embryonic cells. Molecular Biology of the Cell 19 (6): 2465–2475.
Xu, J., P.J. Kausalya, D.C. Phua, S.M. Ali, Z. Hossain, and W. Hunziker. 2008. Early embryonic lethality of mice lacking ZO-2, but Not ZO-3, reveals critical and nonredundant roles for individual zonula occludens proteins in mammalian development. Molecular and Cellular Biology 28 (5): 1669–1678.
Adachi, M., A. Inoko, M. Hata, K. Furuse, K. Umeda, M. Itoh, and S. Tsukita. 2006. Normal establishment of epithelial tight junctions in mice and cultured cells lacking expression of ZO-3, a tight-junction MAGUK protein. Molecular and Cellular Biology 26 (23): 9003–9015.
Saitou, M., M. Furuse, H. Sasaki, J.D. Schulzke, M. Fromm, H. Takano, T. Noda, and S. Tsukita. 2000. Complex phenotype of mice lacking occludin, a component of tight junction strands. Molecular Biology of the Cell 11 (12): 4131–4142.
Schulzke, J.D., A.H. Gitter, J. Mankertz, S. Spiegel, U. Seidler, S. Amasheh, M. Saitou, S. Tsukita, and M. Fromm. 2005. Epithelial transport and barrier function in occludin-deficient mice. Biochimica et Biophysica Acta 1669 (1): 34–42.
Ikenouchi, J., H. Sasaki, S. Tsukita, M. Furuse, and S. Tsukita. 2008. Loss of occludin affects tricellular localization of tricellulin. Molecular Biology of the Cell 19 (11): 4687–4693.
Kitajiri, S., T. Katsuno, H. Sasaki, J. Ito, M. Furuse, and S. Tsukita. 2014. Deafness in occludin-deficient mice with dislocation of tricellulin and progressive apoptosis of the hair cells. Biology Open 3 (8): 759–766.
Pirot, N., H. Delpech, V. Deleuze, C. Dohet, M. Courtade-Saidi, C. Basset-Leobon, E. Chalhoub, D. Mathieu, and V. Pinet. 2014. Lung endothelial barrier disruption in Lyl1-deficient mice. American Journal of Physiology. Lung Cellular and Molecular Physiology 306 (8): L775-785.
Charrier, L.E., E. Loie, and P. Laprise. 2015. Mouse Crumbs3 sustains epithelial tissue morphogenesis in vivo. Scientific Reports 5: 17699.
Wyman, A.E., T.T.T. Nguyen, P. Karki, M.E. Tulapurkar, C.O. Zhang, J. Kim, T.G. Feng, A.J. Dabo, N.W. Todd, I.G. Luzina, P. Geraghty, R.F. Foronjy, J.D. Hasday, A.A. Birukova, S.P. Atamas, and K.G. Birukov. 2020. SIRT7 deficiency suppresses inflammation, induces EndoMT, and increases vascular permeability in primary pulmonary endothelial cells. Scientific Reports 10 (1): 12497.
Fanelli, V., A. Vlachou, S. Ghannadian, U. Simonetti, A.S. Slutsky, and H. Zhang. 2013. Acute respiratory distress syndrome: New definition, current and future therapeutic options. Journal of Thoracic Disease 5 (3): 326–334.
Bernard, G.R., A. Artigas, K.L. Brigham, J. Carlet, K. Falke, L. Hudson, M. Lamy, J.R. Legall, A. Morris, and R. Spragg. 1994. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. American Journal of Respiratory and Critical Care Medicine 149(3 Pt 1): 818–824.
Ganesan, S., A.T. Comstock, and U.S. Sajjan. 2013. Barrier function of airway tract epithelium. Tissue Barriers. 1 (4): e24997.
Denker, B.M., and S.K. Nigam. 1998. Molecular structure and assembly of the tight junction. The American Journal of Physiology 274 (1): F1–9.
Weber, C.R. 2012. Dynamic properties of the tight junction barrier. Annals of the New York Academy of Sciences 1257: 77–84.
Tervonen, A., T.O. Ihalainen, S. Nymark, and J. Hyttinen. 2019. Structural dynamics of tight junctions modulate the properties of the epithelial barrier. PLoS OnE 14 (4): e0214876.
Kachar, B., and T.S. Reese. 1982. Evidence for the lipidic nature of tight junction strands. Nature 296 (5856): 464–466.
Francis, S.A., J.M. Kelly, J. McCormack, R.A. Rogers, J. Lai, E.E. Schneeberger, and R.D. Lynch. 1999. Rapid reduction of MDCK cell cholesterol by methyl-beta-cyclodextrin alters steady state transepithelial electrical resistance. European Journal of Cell Biology 78 (7): 473–484.
Nusrat, A., C.A. Parkos, P. Verkade, C.S. Foley, T.W. Liang, W. Innis-Whitehouse, K.K. Eastburn, and J.L. Madara. 2000. Tight junctions are membrane microdomains. Journal of Cell Science 113 (Pt 10): 1771–1781.
Van Itallie, C.M., A.S. Fanning, J. Holmes, and J.M. Anderson. 2010. Occludin is required for cytokine-induced regulation of tight junction barriers. Journal of Cell Science 123 (Pt 16): 2844–2852.
Marchiando, A.M., L. Shen, W.V. Graham, C.R. Weber, B.T. Schwarz, J.R. Austin 2nd., D.R. Raleigh, Y. Guan, A.J. Watson, M.H. Montrose, and J.R. Turner. 2010. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. The Journal of Cell Biology 189 (1): 111–126.
Terry, S., M. Nie, K. Matter, and M.S. Balda. 2010. Rho signaling and tight junction functions. Physiology (Bethesda, Md.) 25 (1): 16–26.
Gonzalez-Mariscal, L., R. Tapia, and D. Chamorro. 2008. Crosstalk of tight junction components with signaling pathways. Biochimica et Biophysica Acta 1778 (3): 729–756.
Antonov, A., C. Snead, B. Gorshkov, G.N. Antonova, A.D. Verin, and J.D. Catravas. 2008. Heat shock protein 90 inhibitors protect and restore pulmonary endothelial barrier function. American Journal of Respiratory Cell and Molecular Biology 39 (5): 551–559.
Dudek, S.M., and J.G. Garcia. 2001. Cytoskeletal regulation of pulmonary vascular permeability. Journal of Applied Physiology 91(4): 1487–1500.
Yang, M., X.M. Chen, X.G. Du, F.F. Cao, S. Vijaya Luxmi, and Q. Shen. 2013. Continuous blood purification ameliorates endothelial hyperpermeability in SAP patients with MODS by regulating tight junction proteins via ROCK. The International Journal of Artificial Organs 36 (10): 700–709.
Schnoor, M., A. Garcia Ponce, E. Vadillo, R. Pelayo, J. Rossaint, and A. Zarbock. 2017. Actin dynamics in the regulation of endothelial barrier functions and neutrophil recruitment during endotoxemia and sepsis. Cellular and Molecular Life Sciences 74 (11): 1985–1997.
Eutamene, H., V. Theodorou, F. Schmidlin, V. Tondereau, R. Garcia-Villar, C. Salvador-Cartier, M. Chovet, C. Bertrand, and L. Bueno. 2005. LPS-induced lung inflammation is linked to increased epithelial permeability: Role of MLCK. The European Respiratory Journal 25 (5): 789–796.
Petecchia, L., F. Sabatini, C. Usai, E. Caci, L. Varesio, and G.A. Rossi. 2012. Cytokines induce tight junction disassembly in airway cells via an EGFR-dependent MAPK/ERK1/2-pathway. Laboratory Investigation; a Journal of Technical Methods and Pathology 92 (8): 1140–1148.
Hu, Y., J. Lou, Y.Y. Mao, T.W. Lai, L.Y. Liu, C. Zhu, C. Zhang, J. Liu, Y.Y. Li, F. Zhang, W. Li, S.M. Ying, Z.H. Chen, and H.H. Shen. 2016. Activation of MTOR in pulmonary epithelium promotes LPS-induced acute lung injury. Autophagy 12 (12): 2286–2299.
Liu, M., C. Gu, and Y. Wang. 2014. Upregulation of the tight junction protein occludin: Effects on ventilation-induced lung injury and mechanisms of action. BMC Pulmonary Medicine 14: 94.
Xu, S., X. Xue, K. You, and J. Fu. 2016. Caveolin-1 regulates the expression of tight junction proteins during hyperoxia-induced pulmonary epithelial barrier breakdown. Respiratory Research 17 (1): 50.
Birukova, A.A., F. Meng, Y. Tian, A. Meliton, N. Sarich, L.A. Quilliam, and K.G. Birukov. 2015. Prostacyclin post-treatment improves LPS-induced acute lung injury and endothelial barrier recovery via Rap1. Biochimica et Biophysica Acta 1852 (5): 778–791.
Cavanaugh, K.J., Jr., J. Oswari, and S.S. Margulies. 2001. Role of stretch on tight junction structure in alveolar epithelial cells. American Journal of Respiratory Cell and Molecular Biology 25 (5): 584–591.
Kast, J.I., A.J. McFarlane, A. Globinska, M. Sokolowska, P. Wawrzyniak, M. Sanak, J. Schwarze, C.A. Akdis, and K. Wanke. 2017. Respiratory syncytial virus infection influences tight junction integrity. Clinical and Experimental Immunology 190 (3): 351–359.
Kalsi, K.K., J.P. Garnett, W. Patkee, A. Weekes, M.E. Dockrell, E.H. Baker, and D.L. Baines. 2019. Metformin attenuates the effect of Staphylococcus aureus on airway tight junctions by increasing PKCzeta-mediated phosphorylation of occludin. Journal of Cellular and Molecular Medicine 23 (1): 317–327.
Shepley-McTaggart, A., C.A. Sagum, I. Oliva, E. Rybakovsky, K. DiGuilio, J. Liang, M.T. Bedford, J. Cassel, M. Sudol, J.M. Mullin, and R.N. Harty. 2021. SARS-CoV-2 envelope (E) protein interacts with PDZ-domain-2 of host tight junction protein ZO1. PLoS One 16 (6): e0251955.
Sajjan, U., Q. Wang, Y. Zhao, D.C. Gruenert, and M.B. Hershenson. 2008. Rhinovirus disrupts the barrier function of polarized airway epithelial cells. American Journal of Respiratory and Critical Care Medicine 178 (12): 1271–1281.
Capaldo, C.T., and A. Nusrat. 2009. Cytokine regulation of tight junctions. Biochimica et Biophysica Acta 1788 (4): 864–871.
Soini, Y. 2011. Claudins in lung diseases. Respiratory Research 12: 70.
Dolinay, T., B.E. Himes, M. Shumyatcher, G.G. Lawrence, and S.S. Margulies. 2017. Integrated stress response mediates epithelial injury in mechanical ventilation. American Journal of Respiratory Cell and Molecular Biology 57 (2): 193–203.
Gu, C., M. Liu, T. Zhao, D. Wang, and Y. Wang. 2015. Protective role of p120-catenin in maintaining the integrity of adherens and tight junctions in ventilator-induced lung injury. Respiratory Research 16 (1): 58.
Dipaolo, B.C., N. Davidovich, M.G. Kazanietz, and S.S. Margulies. 2013. Rac1 pathway mediates stretch response in pulmonary alveolar epithelial cells. American Journal of Physiology. Lung Cellular and Molecular Physiology 305 (2): L141-153.
Ferreira, A.S., I.N. Silva, F. Fernandes, R. Pilkington, M. Callaghan, S. McClean, and L.M. Moreira. 2015. The tyrosine kinase BceF and the phosphotyrosine phosphatase BceD of Burkholderia contaminans are required for efficient invasion and epithelial disruption of a cystic fibrosis lung epithelial cell line. Infection and Immunity 83 (2): 812–821.
Patkee, W.R., G. Carr, E.H. Baker, D.L. Baines, and J.P. Garnett. 2016. Metformin prevents the effects of Pseudomonas aeruginosa on airway epithelial tight junctions and restricts hyperglycaemia-induced bacterial growth. Journal of Cellular and Molecular Medicine 20 (4): 758–764.
Azghani, A.O., E.J. Miller, and B.T. Peterson. 2000. Virulence factors from Pseudomonas aeruginosa increase lung epithelial permeability. Lung 178 (5): 261–269.
Azghani, A.O. 1996. Pseudomonas aeruginosa and epithelial permeability: Role of virulence factors elastase and exotoxin A. American Journal of Respiratory Cell and Molecular Biology 15 (1): 132–140.
Langer, M., E.S. Duggan, J.L. Booth, V.I. Patel, R.A. Zander, R. Silasi-Mansat, V. Ramani, T.Z. Veres, F. Prenzler, K. Sewald, D.M. Williams, K.M. Coggeshall, S. Awasthi, F. Lupu, D. Burian, J.D. Ballard, A. Braun, and J.P. Metcalf. 2012. Bacillus anthracis lethal toxin reduces human alveolar epithelial barrier function. Infection and Immunity 80 (12): 4374–4387.
Linfield, D.T., A. Raduka, M. Aghapour, and F. Rezaee. 2021. Airway tight junctions as targets of viral infections. Tissue Barriers. 9 (2): 1883965.
Teoh, K.T., Y.L. Siu, W.L. Chan, M.A. Schluter, C.J. Liu, J.S. Peiris, R. Bruzzone, B. Margolis, and B. Nal. 2010. The SARS coronavirus E protein interacts with PALS1 and alters tight junction formation and epithelial morphogenesis. Molecular Biology of the Cell 21 (22): 3838–3852.
Excoffon, K.J., N.D. Gansemer, M.E. Mobily, P.H. Karp, K.R. Parekh, and J. Zabner. 2010. Isoform-specific regulation and localization of the coxsackie and adenovirus receptor in human airway epithelia. PLoS One 5 (3): e9909.
Gonzalez-Juarbe, N., K.M. Bradley, A.T. Shenoy, R.P. Gilley, L.F. Reyes, C.A. Hinojosa, M.I. Restrepo, P.H. Dube, M.A. Bergman, and C.J. Orihuela. 2017. Pore-forming toxin-mediated ion dysregulation leads to death receptor-independent necroptosis of lung epithelial cells during bacterial pneumonia. Cell Death and Differentiation 24 (5): 917–928.
Wynne, B.M., L. Zou, V. Linck, R.S. Hoover, H.P. Ma, and D.C. Eaton. 2017. Regulation of lung epithelial sodium channels by cytokines and chemokines. Frontiers in Immunology 8: 766.
Chambers, R.C., and P.F. Mercer. 2015. Mechanisms of alveolar epithelial injury, repair, and fibrosis. Annals of the American Thoracic Society 12 (Suppl 1): S16-20.
Pugin, J., B. Ricou, K.P. Steinberg, P.M. Suter, and T.R. Martin. 1996. Proinflammatory activity in bronchoalveolar lavage fluids from patients with ARDS, a prominent role for interleukin-1. American Journal of Respiratory and Critical Care Medicine 153 (6 Pt 1): 1850–1856.
Souza-Fernandes, A.B., P. Pelosi, and P.R. Rocco. 2006. Bench-to-bedside review: The role of glycosaminoglycans in respiratory disease. Critical Care 10 (6): 237.
Mazzon, E., and S. Cuzzocrea. 2007. Role of TNF-alpha in lung tight junction alteration in mouse model of acute lung inflammation. Respiratory Research 8 (1): 75.
Marcos-Ramiro, B., D. Garcia-Weber, and J. Millan. 2014. TNF-induced endothelial barrier disruption: Beyond actin and Rho. Thrombosis and Haemostasis 112 (6): 1088–1102.
Herrero, R., L. Prados, A. Ferruelo, F. Puig, R. Pandolfi, R. Guillamat-Prats, L. Moreno, G. Matute-Bello, A. Artigas, A. Esteban, and J. Lorente. 2019. Fas activation alters tight junction proteins in acute lung injury. Thorax 74 (1): 69–82.
Wang, L., and S.M. Dudek. 2009. Regulation of vascular permeability by sphingosine 1-phosphate. Microvascular Research 77 (1): 39–45.
Yang, J., Y. Wang, H. Liu, J. Bi, and Y. Lu. 2017. C2-ceramide influences alveolar epithelial barrier function by downregulating Zo-1, occludin and claudin-4 expression. Toxicology Mechanisms and Methods 27 (4): 293–297.
Vivekananda, J., D. Smith, and R.J. King. 2001. Sphingomyelin metabolites inhibit sphingomyelin synthase and CTP:Phosphocholine cytidylyltransferase. American Journal of Physiology. Lung Cellular and Molecular Physiology 281 (1): L98–L107.
Awasthi, S., J. Vivekananda, V. Awasthi, D. Smith, and R.J. King. 2001. CTP:Phosphocholine cytidylyltransferase inhibition by ceramide via PKC-alpha, p38 MAPK, cPLA2, and 5-lipoxygenase. American Journal of Physiology. Lung Cellular and Molecular Physiology 281 (1): L108-118.
Wittekindt, O.H. 2017. Tight junctions in pulmonary epithelia during lung inflammation. Pflugers Archiv. European Journal of Physiology 469 (1): 135–147.
Türkeli, A., Ö. Yilmaz, M. Karaman, E.T. Kanik, F. Firinci, S. İnan, and H. Yüksel. 2021. Anti-VEGF treatment suppresses remodeling factors and restores epithelial barrier function through the E-cadherin/β-catenin signaling axis in experimental asthma models. Experimental and Therapeutic Medicine 22 (1): 689.
Ganter, M.T., J. Roux, B. Miyazawa, M. Howard, J.A. Frank, G. Su, D. Sheppard, S.M. Violette, P.H. Weinreb, G.S. Horan, M.A. Matthay, and J.F. Pittet. 2008. Interleukin-1beta causes acute lung injury via alphavbeta5 and alphavbeta6 integrin-dependent mechanisms. Circulation Research 102 (7): 804–812.
Ahdieh, M., T. Vandenbos, and A. Youakim. 2001. Lung epithelial barrier function and wound healing are decreased by IL-4 and IL-13 and enhanced by IFN-gamma. American Journal of Physiology. Cell Physiology 281 (6): C2029-2038.
Su, K.C., Y.C. Wu, C.S. Chen, M.H. Hung, Y.H. Hsiao, C.M. Tseng, S.C. Chang, Y.C. Lee, and D.W. Perng. 2013. Bile acids increase alveolar epithelial permeability via mitogen-activated protein kinase, cytosolic phospholipase A2, cyclooxygenase-2, prostaglandin E2 and junctional proteins. Respirology 18 (5): 848–856.
Di, A., D. Mehta, and A.B. Malik. 2016. ROS-activated calcium signaling mechanisms regulating endothelial barrier function. Cell Calcium 60 (3): 163–171.
Mambetsariev, N., T. Mirzapoiazova, B. Mambetsariev, S. Sammani, F.E. Lennon, J.G. Garcia, and P.A. Singleton. 2010. Hyaluronic acid binding protein 2 is a novel regulator of vascular integrity. Arteriosclerosis, Thrombosis, and Vascular Biology 30 (3): 483–490.
Sun, S., T. Sursal, Y. Adibnia, C. Zhao, Y. Zheng, H. Li, L.E. Otterbein, C.J. Hauser, and K. Itagaki. 2013. Mitochondrial DAMPs increase endothelial permeability through neutrophil dependent and independent pathways. PLoS One 8 (3): e59989.
Ward, C., B. Schlingmann, A.A. Stecenko, D.M. Guidot, and M. Koval. 2015. NF-κB inhibitors impair lung epithelial tight junctions in the absence of inflammation. Tissue Barriers. 3 (1–2): e982424.
Coyne, C.B., M.K. Vanhook, T.M. Gambling, J.L. Carson, R.C. Boucher, and L.G. Johnson. 2002. Regulation of airway tight junctions by proinflammatory cytokines. Molecular Biology of the Cell 13 (9): 3218–3234.
Hardyman, M.A., E. Wilkinson, E. Martin, N.P. Jayasekera, C. Blume, E.J. Swindle, N. Gozzard, S.T. Holgate, P.H. Howarth, D.E. Davies, and J.E. Collins. 2013. TNF-alpha-mediated bronchial barrier disruption and regulation by src-family kinase activation. The Journal of Allergy and Clinical Immunology 132(3): 665–675 e668.
Shen, L., C.R. Weber, D.R. Raleigh, D. Yu, and J.R. Turner. 2011. Tight junction pore and leak pathways: A dynamic duo. Annual Review of Physiology 73: 283–309.
Frank, J.A. 2012. Claudins and alveolar epithelial barrier function in the lung. Annals of the New York Academy of Sciences 1257: 175–183.
Schlingmann, B., S.A. Molina, and M. Koval. 2015. Claudins: Gatekeepers of lung epithelial function. Seminars in Cell & Developmental Biology 42: 47–57.
Birukova, A.A., E. Alekseeva, A. Mikaelyan, and K.G. Birukov. 2007. HGF attenuates thrombin-induced endothelial permeability by Tiam1-mediated activation of the Rac pathway and by Tiam1/Rac-dependent inhibition of the Rho pathway. The FASEB Journal 21 (11): 2776–2786.
Birukova, A.A., N. Moldobaeva, J. **ng, and K.G. Birukov. 2008. Magnitude-dependent effects of cyclic stretch on HGF- and VEGF-induced pulmonary endothelial remodeling and barrier regulation. American Journal of Physiology. Lung Cellular and Molecular Physiology 295 (4): L612-623.
Vivekananda, J., V. Awasthi, S. Awasthi, D.B. Smith, and R.J. King. 2000. Hepatocyte growth factor is elevated in chronic lung injury and inhibits surfactant metabolism. American Journal of Physiology. Lung Cellular and Molecular Physiology 278 (2): L382-392.
Hadden, H., S.J. Soldin, and D. Massaro. 2012. Circadian disruption alters mouse lung clock gene expression and lung mechanics. Journal of Applied Physiology 113(3): 385–392.
Mortola, J.P., and E.L. Seifert. 2002. Circadian patterns of breathing. Respiratory Physiology & Neurobiology 131 (1–2): 91–100.
Spengler, C.M., and S.A. Shea. 2000. Endogenous circadian rhythm of pulmonary function in healthy humans. American Journal of Respiratory and Critical Care Medicine 162 (3 Pt 1): 1038–1046.
Chinnapaiyan, S., R.K. Dutta, D. Devadoss, H.S. Chand, I. Rahman, and H.J. Unwalla. 2020. Role of non-coding RNAs in lung circadian clock related diseases. International Journal of Molecular Sciences 21 (8): 3013.
Wu, X., I.S.T. Bos, T.M. Conlon, M. Ansari, V. Verschut, L. van der Koog, L.A. Verkleij, A. D'Ambrosi, A. Matveyenko, H.B. Schiller, H. Konigshoff, M. Schmidt, L.E.M. Kistemaker, A.O. Yildirim, and R. Gosens. 2022. A transcriptomics-guided drug target discovery strategy identifies receptor ligands for lung regeneration. Science Advances 8 (12): eabj9949.
Hwang, J.W., I.K. Sundar, H. Yao, M.T. Sellix, and I. Rahman. 2014. Circadian clock function is disrupted by environmental tobacco/cigarette smoke, leading to lung inflammation and injury via a SIRT1-BMAL1 pathway. The FASEB Journal 28 (1): 176–194.
Chen, H.C., Y.C. Chen, T.N. Wang, W.F. Fang, Y.C. Chang, Y.M. Chen, I.Y. Chen, M.C. Lin, and M.Y. Yang. 2021. Disrupted expression of circadian clock genes in patients with bronchial asthma. Journal of Asthma and Allergy 14: 371–380.
Nakao, A. 2020. Circadian regulation of the biology of allergic disease: Clock disruption can promote allergy. Frontiers in Immunology 11: 1237.
Lagishetty, V., P.T. Parthasarathy, O. Phillips, J. Fukumoto, Y. Cho, I. Fukumoto, H. Bao, R. Cox Jr., L. Galam, R.F. Lockey, and N. Kolliputi. 2014. Dysregulation of CLOCK gene expression in hyperoxia-induced lung injury. American Journal of Physiology. Cell Physiology 306 (11): C999–C1007.
Oyama, Y., S.R. Shuff, N. Burns, C.U. Vohwinkel, and T. Eckle. 2022. Intense light-elicited alveolar type 2-specific circadian PER2 protects from bacterial lung injury via BPIFB1. American Journal of Physiology. Lung Cellular and Molecular Physiology 322 (5): L647–L661.
Zhuang, X., S. Tsukuda, F. Wrensch, P.A.C. Wing, M. Schilling, J.M. Harris, H. Borrmann, S.B. Morgan, J.L. Cane, L. Mailly, N. Thakur, C. Conceicao, H. Sanghani, L. Heydmann, C. Bach, A. Ashton, S. Walsh, T.K. Tan, L. Schimanski, K.A. Huang, C. Schuster, K. Watashi, T.S.C. Hinks, A. Jagannath, S.R. Vausdevan, D. Bailey, T.F. Baumert, and J.A. McKeating. 2021. The circadian clock component BMAL1 regulates SARS-CoV-2 entry and replication in lung epithelial cells. iScience 24 (10): 103144.
Meira, E.C.M., M. Miyazawa, and D. Gozal. 2020. Putative contributions of circadian clock and sleep in the context of SARS-CoV-2 infection. The European Respiratory Journal 55 (6): 2001023.
Cunningham, P.S., P. Meijer, A. Nazgiewicz, S.G. Anderson, L.A. Borthwick, J. Bagnall, G.B. Kitchen, M. Lodyga, N. Begley, R.V. Venkateswaran, R. Shah, P.F. Mercer, H.J. Durrington, N.C. Henderson, K. Piper-Hanley, A.J. Fisher, R.C. Chambers, D.A. Bechtold, J.E. Gibbs, A.S. Loudon, M.K. Rutter, B. Hinz, D.W. Ray, and J.F. Blaikley. 2020. The circadian clock protein REVERBalpha inhibits pulmonary fibrosis development. Proceedings of the National Academy of Sciences of the United States of America 117 (2): 1139–1147.
Gibbs, J., L. Ince, L. Matthews, J. Mei, T. Bell, N. Yang, B. Saer, N. Begley, T. Poolman, M. Pariollaud, S. Farrow, F. DeMayo, T. Hussell, G.S. Worthen, D. Ray, and A. Loudon. 2014. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nature Medicine 20 (8): 919–926.
Wang, Y., P. Pati, Y. Xu, F. Chen, D.W. Stepp, Y. Huo, R.D. Rudic, and D.J. Fulton. 2016. Endotoxin disrupts circadian rhythms in macrophages via reactive oxygen species. PLoS One 11 (5): e0155075.
Sundar, I.K., H. Yao, M.T. Sellix, and I. Rahman. 2015. Circadian molecular clock in lung pathophysiology. American Journal of Physiology. Lung Cellular and Molecular Physiology 309 (10): L1056-1075.
Cheng, F.L., Y.F. An, J.M. Xue, Y.J. Wang, X.W. Ding, Y.T. Zhang, and C.Q. Zhao. 2022. Circadian rhythm disruption exacerbates Th2-like immune response in murine allergic airway inflammation. International Forum of Allergy and Rhinology 12 (5): 757–770.
**ng, C., Y. Zhou, H. Xu, M. Ding, Y. Zhang, M. Zhang, M. Hu, X. Huang, and L. Song. 2021. Sleep disturbance induces depressive behaviors and neuroinflammation by altering the circadian oscillations of clock genes in rats. Neuroscience Research 171: 124–132.
Kyoko, O.O., H. Kono, K. Ishimaru, K. Miyake, T. Kubota, H. Ogawa, K. Okumura, S. Shibata, and A. Nakao. 2014. Expressions of tight junction proteins occludin and claudin-1 are under the circadian control in the mouse large intestine: Implications in intestinal permeability and susceptibility to colitis. PLoS One 9 (5): e98016.
Yamato, M., T. Ito, H. Iwatani, M. Yamato, E. Imai, and H. Rakugi. 2010. E-cadherin and claudin-4 expression has circadian rhythm in adult rat kidney. Journal of Nephrology 23 (1): 102–110.
Hudson, N., L. Celkova, A. Hopkins, C. Greene, F. Storti, E. Ozaki, E. Fahey, S. Theodoropoulou, P.F. Kenna, M.M. Humphries, A.M. Curtis, E. Demmons, A. Browne, S. Liddie, M.S. Lawrence, C. Grimm, M.T. Cahill, P. Humphries, S.L. Doyle, and M. Campbell. 2019. Dysregulated claudin-5 cycling in the inner retina causes retinal pigment epithelial cell atrophy. JCI Insight 4 (15): e130273.
Kleeberger, S.R., S. Reddy, L.Y. Zhang, and A.E. Jedlicka. 2000. Genetic susceptibility to ozone-induced lung hyperpermeability: Role of toll-like receptor 4. American Journal of Respiratory Cell and Molecular Biology 22 (5): 620–627.
Kleeberger, S.R., S.P. Reddy, L.Y. Zhang, H.Y. Cho, and A.E. Jedlicka. 2001. Toll-like receptor 4 mediates ozone-induced murine lung hyperpermeability via inducible nitric oxide synthase. American Journal of Physiology. Lung Cellular and Molecular Physiology 280 (2): L326-333.
Carrington, J.M., and J.A. Poole. 2018. The effect of inhalant organic dust on bone health. Current Allergy and Asthma Reports 18 (3): 16.
Hussain, S., C.G. Johnson, J. Sciurba, X. Meng, V.P. Stober, C. Liu, J.M. Cyphert-Daly, K. Bulek, W. Qian, A. Solis, Y. Sakamachi, C.S. Trempus, J.J. Aloor, K.M. Gowdy, W.M. Foster, J.W. Hollingsworth, R.M. Tighe, X. Li, M.B. Fessler, and S. Garantziotis. 2020. TLR5 participates in the TLR4 receptor complex and promotes MyD88-dependent signaling in environmental lung injury. eLife 9: e50458.
Gilmour, P.S., M.C. Schladweiler, J.H. Richards, A.D. Ledbetter, and U.P. Kodavanti. 2004. Hypertensive rats are susceptible to TLR4-mediated signaling following exposure to combustion source particulate matter. Inhalation Toxicology 16 (Suppl 1): 5–18.
Chun, C.D., W.C. Liles, C.W. Frevert, R.W. Glenny, and W.A. Altemeier. 2010. Mechanical ventilation modulates toll-like receptor-3-induced lung inflammation via a MyD88-dependent, TLR4-independent pathway: A controlled animal study. BMC Pulmonary Medicine 10: 57.
Armstrong, L., A.R. Medford, K.M. Up**ton, J. Robertson, I.R. Witherden, T.D. Tetley, and A.B. Millar. 2004. Expression of functional toll-like receptor-2 and -4 on alveolar epithelial cells. American Journal of Respiratory Cell and Molecular Biology 31 (2): 241–245.
Sodhi, C.P., H. Jia, Y. Yamaguchi, P. Lu, M. Good, C. Egan, J. Ozolek, X. Zhu, T.R. Billiar, and D.J. Hackam. 2015. Intestinal epithelial TLR-4 activation is required for the development of acute lung injury after trauma/hemorrhagic shock via the release of HMGB1 from the gut. The Journal of Immunology 194 (10): 4931–4939.
Chakraborty, D., S. Zenker, J. Rossaint, A. Holscher, M. Pohlen, A. Zarbock, J. Roth, and T. Vogl. 2017. Alarmin S100A8 activates alveolar epithelial cells in the context of acute lung injury in a TLR4-dependent manner. Frontiers in Immunology 8: 1493.
Chen, X.X., L. Tang, Z.H. Han, W.J. Wang, and J.G. Meng. 2019. Coculture with bone marrow-derived mesenchymal stem cells attenuates inflammation and apoptosis in lipopolysaccharide-stimulated alveolar epithelial cells via enhanced secretion of keratinocyte growth factor and angiopoietin-1 modulating the Toll-like receptor-4 signal pathway. Molecular Medicine Reports 19 (3): 1891–1902.
Pastor, C.M., J. Pugin, B. Kwak, M. Chanson, F. Mach, A. Hadengue, and J.L. Frossard. 2004. Role of toll-like receptor 4 on pancreatic and pulmonary injury in a mice model of acute pancreatitis associated with endotoxemia. Critical Care Medicine 32 (8): 1759–1763.
Zhang, Y.P., C.S. Pan, L. Yan, Y.Y. Liu, B.H. Hu, X. Chang, Q. Li, D.D. Huang, H.Y. Sun, G. Fu, K. Sun, J.Y. Fan, and J.Y. Han. 2016. Catalpol restores LPS-elicited rat microcirculation disorder by regulation of a network of signaling involving inhibition of TLR-4 and SRC. American Journal of Physiology. Gastrointestinal and Liver Physiology 311 (6): G1091-g1104.
Peng, L.Y., M. Yuan, H.T. Shi, J.H. Li, K. Song, J.N. Huang, P.F. Yi, B.D. Fu, and H.Q. Shen. 2019. Protective effect of piceatannol against acute lung injury through protecting the integrity of air-blood barrier and modulating the TLR4/NF-κB signaling pathway activation. Frontiers in Pharmacology 10: 1613.
Li, X., R. Li, Q. Fang, M. Jamal, C. Wang, Y. Wang, Z. Zhang, X. Wu, and X. Song. 2021. Oxycodone attenuates vascular leak and lung inflammation in a clinically relevant two-hit rat model of acute lung injury. Cytokine 138: 155346.
Peng, L.Y., H.T. Shi, M. Yuan, J.H. Li, K. Song, J.N. Huang, P.F. Yi, H.Q. Shen, and B.D. Fu. 2020. Madecassoside protects against LPS-induced acute lung injury via inhibiting TLR4/NF-κB activation and blood-air barrier permeability. Frontiers in Pharmacology 11: 807.
Zhu, H., X. Lu, L. Ling, H. Li, Y. Ou, X. Shi, Y. Lu, Y. Zhang, and D. Chen. 2018. Houttuynia cordata polysaccharides ameliorate pneumonia severity and intestinal injury in mice with influenza virus infection. Journal of Ethnopharmacology 218: 90–99.
Sun, K., R. Huang, L. Yan, D.T. Li, Y.Y. Liu, X.H. Wei, Y.C. Cui, C.S. Pan, J.Y. Fan, X. Wang, and J.Y. Han. 2018. Schisandrin attenuates lipopolysaccharide-induced lung injury by regulating TLR-4 and Akt/FoxO1 signaling pathways. Frontiers in Physiology 9: 1104.
Ma, L.Q., C.S. Pan, N. Yang, Y.Y. Liu, L. Yan, K. Sun, X.H. Wei, K. He, M.M. **ao, J.Y. Fan, and J.Y. Han. 2014. Posttreatment with Ma-**ng-Shi-Gan-Tang, a Chinese medicine formula, ameliorates lipopolysaccharide-induced lung microvessel hyperpermeability and inflammatory reaction in rat. Microcirculation 21 (7): 649–663.
Liu, M.W., Y.H. Wang, C.Y. Qian, and H. Li. 2014. Xuebi**g exerts protective effects on lung permeability leakage and lung injury by upregulating toll-interacting protein expression in rats with sepsis. International Journal of Molecular Medicine 34 (6): 1492–1504.
Herath, K., H.J. Kim, J.H. Lee, J.G. Je, H.S. Yu, Y.J. Jeon, H.J. Kim, and Y. Jee. 2021. Sargassum horneri (Turner) C. Agardh containing polyphenols attenuates particulate matter-induced inflammatory response by blocking TLR-mediated MYD88-dependent MAPK signaling pathway in MLE-12 cells. Journal of Ethnopharmacology 265: 113340.
Zhang, G., X. Zhang, H. Huang, Y. Ji, D. Li, and W. Jiang. 2018. Saquinavir plus methylprednisolone ameliorates experimental acute lung injury. Brazilian Journal of Medical and Biological Research 51 (10): e7579.
Zhao, L., M. Li, K. Sun, S. Su, T. Geng, and H. Sun. 2020. Hippophae rhamnoides polysaccharides protect IPEC-J2 cells from LPS-induced inflammation, apoptosis and barrier dysfunction in vitro via inhibiting TLR4/NF-kappaB signaling pathway. International Journal of Biological Macromolecules 155: 1202–1215.
Yang, S., L. Li, L. Yu, L. Sun, K. Li, C. Tong, W. Xu, G. Cui, M. Long, and P. Li. 2020. Selenium-enriched yeast reduces caecal pathological injuries and intervenes changes of the diversity of caecal microbiota caused by ochratoxin-A in broilers. Food and Chemical Toxicology: an International Journal Published for the British Industrial Biological Research Association 137: 111139.
Meduri, G.U., E. Golden, A.X. Freire, E. Taylor, M. Zaman, S.J. Carson, M. Gibson, and R. Umberger. 2007. Methylprednisolone infusion in early severe ARDS: Results of a randomized controlled trial. Chest 131 (4): 954–963.
Jamaati, H., S.M. Hashemian, B. Farzanegan, M. Malekmohammad, P. Tabarsi, M. Marjani, A. Moniri, Z. Abtahian, S. Haseli, E. Mortaz, A. Dastan, A. Mohamadnia, A. Vahedi, F. Monjazebi, F. Yassari, L. Fadaeizadeh, A. Saffaei, and F. Dastan. 2021. No clinical benefit of high dose corticosteroid administration in patients with COVID-19: A preliminary report of a randomized clinical trial. European Journal of Pharmacology 897: 173947.
Steinberg, K.P., L.D. Hudson, R.B. Goodman, C.L. Hough, P.N. Lanken, R. Hyzy, B.T. Thompson, M. Ancukiewicz, and L. National Heart. 2006. Blood Institute Acute Respiratory Distress Syndrome Clinical Trials N. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. The New England Journal of Medicine 354(16): 1671–1684.
Tongyoo, S., C. Permpikul, W. Mongkolpun, V. Vattanavanit, S. Udompanturak, M. Kocak, and G.U. Meduri. 2016. Hydrocortisone treatment in early sepsis-associated acute respiratory distress syndrome: Results of a randomized controlled trial. Critical Care 20 (1): 329.
Annane, D., V. Sébille, and E. Bellissant. 2006. Effect of low doses of corticosteroids in septic shock patients with or without early acute respiratory distress syndrome. Critical Care Medicine 34 (1): 22–30.
Meduri, G.U., E.A. Tolley, G.P. Chrousos, and F. Stentz. 2002. Prolonged methylprednisolone treatment suppresses systemic inflammation in patients with unresolving acute respiratory distress syndrome: Evidence for inadequate endogenous glucocorticoid secretion and inflammation-induced immune cell resistance to glucocorticoids. American Journal of Respiratory and Critical Care Medicine 165 (7): 983–991.
Schwartz, H.J., F.C. Lowell, and J.C. Melby. 1968. Steroid resistance in bronchial asthma. Annals of Internal Medicine 69 (3): 493–499.
Steinberg, K.P., L.D. Hudson, R.B. Goodman, C.L. Hough, P.N. Lanken, R. Hyzy, B.T. Thompson, and M. Ancukiewicz. 2006. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. The New England Journal of Medicine 354 (16): 1671–1684.
Zhang, Z., L. Chen, and H. Ni. 2015. The effectiveness of corticosteroids on mortality in patients with acute respiratory distress syndrome or acute lung injury: A secondary analysis. Scientific Reports 5: 17654.
den Otter, J.J., C.P. van Schayck, H.T. Folgering, G. van den Boom, R.P. Akkermans, and C. van Weel. 2007. Early intervention with inhaled corticosteroids in subjects with rapid decline in lung function and signs of bronchial hyperresponsiveness: Results from the DIMCA programme. The European Journal of General Practice 13 (2): 89–91.
Grünberg, K., R.F. Sharon, J.K. Sont, J.C. In‘t Veen, W.A. Van Schadewijk, E.P. De Klerk, C.R. Dick, J.H. Van Krieken, and P.J. Sterk. 2001. Rhinovirus-induced airway inflammation in asthma: effect of treatment with inhaled corticosteroids before and during experimental infection. American Journal of Respiratory and Critical Care Medicine 164(10 Pt 1): 1816–1822.
Vähätalo, I., P. Ilmarinen, L.E. Tuomisto, O. Niemelä, and H. Kankaanranta. 2018. Inhaled corticosteroids and asthma control in adult-onset asthma: 12-year follow-up study. Respiratory Medicine 137: 70–76.
Stahn, C., M. Lowenberg, D.W. Hommes, and F. Buttgereit. 2007. Molecular mechanisms of glucocorticoid action and selective glucocorticoid receptor agonists. Molecular and Cellular Endocrinology 275 (1–2): 71–78.
Buttgereit, F., K.G. Saag, M. Cutolo, J.A. da Silva, and J.W. Bijlsma. 2005. The molecular basis for the effectiveness, toxicity, and resistance to glucocorticoids: Focus on the treatment of rheumatoid arthritis. Scandinavian Journal of Rheumatology 34 (1): 14–21.
Ramamoorthy, S., and J.A. Cidlowski. 2016. Corticosteroids: mechanisms of action in health and disease. Rheumatic Diseases Clinics of North America 42(1): 15–31, vii.
Kim, S.R., and Y.C. Lee. 2015. Endoplasmic reticulum stress and the related signaling networks in severe asthma. Allergy, Asthma & Immunology Research 7 (2): 106–117.
MacRedmond, R.E., C.M. Greene, D.R. Dorscheid, N.G. McElvaney, and S.J. O’Neill. 2007. Epithelial expression of TLR4 is modulated in COPD and by steroids, salmeterol and cigarette smoke. Respiratory Research 8 (1): 84.
Kielgast, F., H. Schmidt, P. Braubach, V.E. Winkelmann, K.E. Thompson, M. Frick, P. Dietl, and O.H. Wittekindt. 2016. Glucocorticoids regulate tight junction permeability of lung epithelia by modulating Claudin 8. American Journal of Respiratory Cell and Molecular Biology 54 (5): 707–717.
Barnes, P.J. 2006. Corticosteroids: The drugs to beat. European Journal of Pharmacology 533 (1–3): 2–14.
Marshall, C.L., K. Hasani, and N. Mookherjee. 2021. Immunobiology of steroid-unresponsive severe asthma. Frontiers in Allergy 2: 718267.
Sousa, A.R., S.J. Lane, J.A. Cidlowski, D.Z. Staynov, and T.H. Lee. 2000. Glucocorticoid resistance in asthma is associated with elevated in vivo expression of the glucocorticoid receptor beta-isoform. The Journal of Allergy and Clinical Immunology 105 (5): 943–950.
Cho, Y.J., and K.E. Lee. 2003. Decreased glucocorticoid binding affinity to glucocorticoid receptor is important in the poor response to steroid therapy of older-aged patients with severe bronchial asthma. Allergy and Asthma Proceedings 24 (5): 353–358.
Kim, R.Y., J.W. Pinkerton, P.G. Gibson, M.A. Cooper, J.C. Horvat, and P.M. Hansbro. 2015. Inflammasomes in COPD and neutrophilic asthma. Thorax 70 (12): 1199–1201.
Kim, R.Y., J.W. Pinkerton, A.T. Essilfie, A.A.B. Robertson, K.J. Baines, A.C. Brown, J.R. Mayall, M.K. Ali, M.R. Starkey, N.G. Hansbro, J.A. Hirota, L.G. Wood, J.L. Simpson, D.A. Knight, P.A. Wark, P.G. Gibson, L.A.J. O’Neill, M.A. Cooper, J.C. Horvat, and P.M. Hansbro. 2017. Role for NLRP3 Inflammasome-mediated, IL-1beta-dependent responses in severe, steroid-resistant asthma. American Journal of Respiratory and Critical Care Medicine 196 (3): 283–297.
Stolberg, V.R., A.L. McCubbrey, C.M. Freeman, J.P. Brown, S.W. Crudgington, S.H. Taitano, B.L. Saxton, P. Mancuso, and J.L. Curtis. 2015. Glucocorticoid-augmented efferocytosis inhibits pulmonary pneumococcal clearance in mice by reducing alveolar macrophage bactericidal function. The Journal of Immunology 195 (1): 174–184.
Crim, C., P.M. Calverley, J.A. Anderson, B. Celli, G.T. Ferguson, C. Jenkins, P.W. Jones, L.R. Willits, J.C. Yates, and J. Vestbo. 2009. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. The European Respiratory Journal 34 (3): 641–647.
Suissa, S., V. Patenaude, F. Lapi, and P. Ernst. 2013. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax 68 (11): 1029–1036.
Goleva, E., L.P. Jackson, J.K. Harris, C.E. Robertson, E.R. Sutherland, C.F. Hall, J.T. Good Jr., E.W. Gelfand, R.J. Martin, and D.Y. Leung. 2013. The effects of airway microbiome on corticosteroid responsiveness in asthma. American Journal of Respiratory and Critical Care Medicine 188 (10): 1193–1201.
Ueda, K., Y. Nishimoto, G. Kimura, T. Masuko, P.J. Barnes, K. Ito, and Y. Kizawa. 2016. Repeated lipopolysaccharide exposure causes corticosteroid insensitive airway inflammation via activation of phosphoinositide-3-kinase delta pathway. Biochemistry and Biophysics Reports 7: 367–373.
Hadebe, S., F. Kirstein, K. Fierens, K. Chen, R.A. Drummond, S. Vautier, S. Sajaniemi, G. Murray, D.L. Williams, P. Redelinghuys, T.A. Reinhart, B.A. Junecko, J.K. Kolls, B.N. Lambrecht, F. Brombacher, and G.D. Brown. 2015. Correction: Microbial ligand costimulation drives neutrophilic steroid-refractory asthma. PLoS One 10 (9): e0137945.
Yang, M., R.K. Kumar, and P.S. Foster. 2009. Pathogenesis of steroid-resistant airway hyperresponsiveness: Interaction between IFN-gamma and TLR4/MyD88 pathways. The Journal of Immunology 182 (8): 5107–5115.
Southworth, T., A. Metryka, S. Lea, S. Farrow, J. Plumb, and D. Singh. 2012. IFN-gamma synergistically enhances LPS signalling in alveolar macrophages from COPD patients and controls by corticosteroid-resistant STAT1 activation. British Journal of Pharmacology 166 (7): 2070–2083.
Hart, L., S. Lim, I. Adcock, P.J. Barnes, and K.F. Chung. 2000. Effects of inhaled corticosteroid therapy on expression and DNA-binding activity of nuclear factor kappaB in asthma. American Journal of Respiratory and Critical Care Medicine 161 (1): 224–231.
Raleigh, D.R., A.M. Marchiando, Y. Zhang, L. Shen, H. Sasaki, Y. Wang, M. Long, and J.R. Turner. 2010. Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlap** functions. Molecular Biology of the Cell 21 (7): 1200–1213.
Smyth, T., J. Veazey, S. Eliseeva, D. Chalupa, A. Elder, and S.N. Georas. 2020. Diesel exhaust particle exposure reduces expression of the epithelial tight junction protein tricellulin. Particle and Fibre Toxicology 17 (1): 52.
Riazuddin, S., Z.M. Ahmed, A.S. Fanning, A. Lagziel, S. Kitajiri, K. Ramzan, S.N. Khan, P. Chattaraj, P.L. Friedman, J.M. Anderson, I.A. Belyantseva, A. Forge, S. Riazuddin, and T.B. Friedman. 2006. Tricellulin is a tight-junction protein necessary for hearing. American Journal of Human Genetics 79 (6): 1040–1051.
Kojima, T., Y. Shindo, T. Konno, Y. Kodera, W. Arai, M. Miyakawa, K. Ohwada, H. Tanaka, M. Tsujiwaki, Y. Sakuma, S. Kikuchi, T. Ohkuni, K. Takano, A. Watanabe, and T. Kohno. 2022. Dysfunction of epithelial permeability barrier induced by HMGB1 in 2.5D cultures of human epithelial cells. Tissue Barriers 10 (2): 1972760.
Zhang, Z.W., A.R. Ansari, L. Dong, X.Y. Niu, W.J. Yang, H.Z. Li, F.L. Xu, K.L. Yang, and H. Song. 2022. Alterations in the expression level of visfatin in the lungs of piglets infected with PRRSV and its effect on PRRSV replication. Microbial Pathogenesis 164: 105443.
Tessema, M., C.M. Yingling, Y. Liu, C.S. Tellez, L. Van Neste, S.S. Baylin, and S.A. Belinsky. 2014. Genome-wide unmasking of epigenetically silenced genes in lung adenocarcinoma from smokers and never smokers. Carcinogenesis 35 (6): 1248–1257.
Kaarteenaho, R., H. Merikallio, S. Lehtonen, T. Harju, and Y. Soini. 2010. Divergent expression of claudin-1, -3, -4, -5 and -7 in develo** human lung. Respiratory Research 11 (1): 59.
Kaarteenaho-Wiik, R., and Y. Soini. 2009. Claudin-1, -2, -3, -4, -5, and -7 in usual interstitial pneumonia and sarcoidosis. Journal of Histochemistry and Cytochemistry: Official Journal of the Histochemistry Society 57 (3): 187–195.
Kostrewa, D., M. Brockhaus, A. D’Arcy, G.E. Dale, P. Nelboeck, G. Schmid, F. Mueller, G. Bazzoni, E. Dejana, T. Bartfai, F.K. Winkler, and M. Hennig. 2001. X-ray structure of junctional adhesion molecule: Structural basis for homophilic adhesion via a novel dimerization motif. The EMBO Journal 20 (16): 4391–4398.
Weber, C., L. Fraemohs, and E. Dejana. 2007. The role of junctional adhesion molecules in vascular inflammation. Nature Reviews. Immunology 7 (6): 467–477.
Torres-Flores, J.M., and C.F. Arias. 2015. Tight junctions go viral! Viruses 7 (9): 5145–5154.
Bauer, H., J. Zweimueller-Mayer, P. Steinbacher, A. Lametschwandtner, and H.C. Bauer. 2010. The dual role of zonula occludens (ZO) proteins. Journal of Biomedicine & Biotechnology 2010: 402593.
Balda, M.S., and K. Matter. 2000. The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. The EMBO Journal 19 (9): 2024–2033.
Betanzos, A., M. Huerta, E. Lopez-Bayghen, E. Azuara, J. Amerena, and L. Gonzalez-Mariscal. 2004. The tight junction protein ZO-2 associates with Jun, Fos and C/EBP transcription factors in epithelial cells. Experimental Cell Research 292 (1): 51–66.
Traweger, A., R. Fuchs, I.A. Krizbai, T.M. Weiger, H.C. Bauer, and H. Bauer. 2003. The tight junction protein ZO-2 localizes to the nucleus and interacts with the heterogeneous nuclear ribonucleoprotein scaffold attachment factor-B. Journal of Biological Chemistry 278 (4): 2692–2700.
**a, W., Z. Pan, H. Zhang, Q. Zhou, and Y. Liu. 2020. Inhibition of ERRalpha aggravates sepsis-induced acute lung injury in rats via provoking inflammation and oxidative stress. Oxidative Medicine and Cellular Longevity 2020: 2048632.
Chen, B., Z. Yang, C. Yang, W. Qin, J. Gu, C. Hu, A. Chen, K. Ning, B. Yi, and K. Lu. 2018. A self-organized actomyosin drives multiple intercellular junction disruption and directly promotes neutrophil recruitment in lipopolysaccharide-induced acute lung injury. The FASEB Journal fj201701506RR.
Gross, C.M., M. Kellner, T. Wang, Q. Lu, X. Sun, E.A. Zemskov, S. Noonepalle, A. Kangath, S. Kumar, M. Gonzalez-Garay, A.A. Desai, S. Aggarwal, B. Gorshkov, C. Klinger, A.D. Verin, J.D. Catravas, J.R. Jacobson, J.X. Yuan, R. Rafikov, J.G.N. Garcia, and S.M. Black. 2018. LPS-induced acute lung injury involves NF-kappaB-mediated downregulation of SOX18. American Journal of Respiratory Cell and Molecular Biology 58 (5): 614–624.
Kling, K.M., E. Lopez-Rodriguez, C. Pfarrer, C. Muhlfeld, and C. Brandenberger. 2017. Aging exacerbates acute lung injury-induced changes of the air-blood barrier, lung function, and inflammation in the mouse. American Journal of Physiology. Lung Cellular and Molecular Physiology 312 (1): L1–L12.
Feng, J., W. Pan, X. Yang, F. Long, J. Zhou, Y. Liao, and M. Wang. 2021. RBM3 increases cell survival but disrupts tight junction of microvascular endothelial cells in acute lung injury. The Journal of Surgical Research 261: 226–235.
Zhang, Y.L., Q.Q. Li, W. Guo, Y. Huang, and J. Yang. 2007. Effects of chronic ethanol ingestion on tight junction proteins and barrier function of alveolar epithelium in the rat. Shock 28 (2): 245–252.
Tang, M., L. Chen, B. Li, Y. Wang, S. Li, A. Wen, S. Yao, and Y. Shang. 2016. BML-111 attenuates acute lung injury in endotoxemic mice. The Journal of Surgical Research 200 (2): 619–630.
Zhao, X., C. Gu, and Y. Wang. 2020. PAD4 selective inhibitor TDFA protects lipopolysaccharide-induced acute lung injury by modulating nuclear p65 localization in epithelial cells. International Immunopharmacology 88: 106923.
Fisher, B.J., D. Kraskauskas, E.J. Martin, D. Farkas, J.A. Wegelin, D. Brophy, K.R. Ward, N.F. Voelkel, A.A. Fowler 3rd., and R. Natarajan. 2012. Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. American Journal of Physiology. Lung Cellular and Molecular Physiology 303 (1): L20-32.
Liu, M.M., J. Zhou, D. Ji, J. Yang, Y.P. Huang, and Q. Wang. 2021. Diammonium glycyrrhizinate lipid ligand ameliorates lipopolysaccharide-induced acute lung injury by modulating vascular endothelial barrier function. Experimental and Therapeutic Medicine 21 (4): 303.
Yao, L., Y. Tang, J. Chen, J. Li, H. Wang, M. Lu, L. Gao, F. Liu, P. Chang, X. Liu, and H. Tang. 2021. Impaired airway epithelial barrier integrity was mediated by PI3Kdelta in a mouse model of lipopolysaccharide-induced acute lung injury. International Immunopharmacology 95: 107570.
Yuan, Z.C., N. Zeng, L. Liu, T. Wang, L.Q. Dai, H. Wang, Z.J. Zeng, Y.F. Cao, Y.F. Zhou, D. Xu, Y.C. Shen, and F.Q. Wen. 2021. Mitochondrial damage-associated molecular patterns exacerbate lung fluid imbalance via the formyl peptide receptor-1 signaling pathway in acute lung injury. Critical Care Medicine 49 (1): e53–e62.
Shan, Y., A. Akram, H. Amatullah, D.Y. Zhou, P.L. Gali, T. Maron-Gutierrez, A. Gonzalez-Lopez, L. Zhou, P.R. Rocco, D. Hwang, G.M. Albaiceta, J.J. Haitsma, and C.C. dos Santos. 2015. ATF3 protects pulmonary resident cells from acute and ventilator-induced lung injury by preventing Nrf2 degradation. Antioxidants & Redox Signaling 22 (8): 651–668.
Meng, F., A. Meliton, N. Moldobaeva, G. Mutlu, Y. Kawasaki, T. Akiyama, and A.A. Birukova. 2015. Asef mediates HGF protective effects against LPS-induced lung injury and endothelial barrier dysfunction. American Journal of Physiology. Lung Cellular and Molecular Physiology 308 (5): L452-463.
Zhou, Y., P. Li, A.J. Goodwin, J.A. Cook, P.V. Halushka, E. Chang, B. Zingarelli, and H. Fan. 2019. Exosomes from endothelial progenitor cells improve outcomes of the lipopolysaccharide-induced acute lung injury. Critical Care 23 (1): 44.
Liu, B., H. Zhao, Y. Wang, H. Zhang, and Y. Ma. 2020. Astragaloside IV attenuates lipopolysaccharides-induced pulmonary epithelial cell unjury through inhibiting autophagy. Pharmacology 105 (1–2): 90–101.
Meng, S.S., F.M. Guo, X.W. Zhang, W. Chang, F. Peng, H.B. Qiu, and Y. Yang. 2019. mTOR/STAT-3 pathway mediates mesenchymal stem cell-secreted hepatocyte growth factor protective effects against lipopolysaccharide-induced vascular endothelial barrier dysfunction and apoptosis. Journal of Cellular Biochemistry 120 (3): 3637–3650.
Li, J., K. Wang, B. Huang, R. Li, X. Wang, H. Zhang, H. Tang, and X. Chen. 2021. The receptor for advanced glycation end products mediates dysfunction of airway epithelial barrier in a lipopolysaccharides-induced murine acute lung injury model. International Immunopharmacology 93: 107419.
Etzrodt, V., T.O. Idowu, H. Schenk, B. Seeliger, A. Prasse, K. Thamm, T. Pape, J. Muller-Deile, M. van Meurs, T. Thum, A. Garg, R. Geffers, K. Stahl, S.M. Parikh, H. Haller, and S. David. 2021. Role of endothelial microRNA 155 on capillary leakage in systemic inflammation. Critical Care (London, England) 25 (1): 76.
Song, M.J., N. Davidovich, G.G. Lawrence, and S.S. Margulies. 2016. Superoxide mediates tight junction complex dissociation in cyclically stretched lung slices. Journal of Biomechanics 49 (8): 1330–1335.
Shen, C.H., J.Y. Lin, C.Y. Lu, S.S. Yang, C.K. Peng, and K.L. Huang. 2021. SPAK-p38 MAPK signal pathway modulates claudin-18 and barrier function of alveolar epithelium after hyperoxic exposure. BMC Pulmonary Medicine 21 (1): 58.
Wray, C., Y. Mao, J. Pan, A. Chandrasena, F. Piasta, and J.A. Frank. 2009. Claudin-4 augments alveolar epithelial barrier function and is induced in acute lung injury. American Journal of Physiology. Lung Cellular and Molecular Physiology 297 (2): L219-227.
You, K., X. Xu, J. Fu, S. Xu, X. Yue, Z. Yu, and X. Xue. 2012. Hyperoxia disrupts pulmonary epithelial barrier in newborn rats via the deterioration of occludin and ZO-1. Respiratory Research 13 (1): 36.
Vyas-Read, S., R.J. Vance, W. Wang, J. Colvocoresses-Dodds, L.A. Brown, and M. Koval. 2018. Hyperoxia induces paracellular leak and alters claudin expression by neonatal alveolar epithelial cells. Pediatric Pulmonology 53 (1): 17–27.
Ohta, H., S. Chiba, M. Ebina, M. Furuse, and T. Nukiwa. 2012. Altered expression of tight junction molecules in alveolar septa in lung injury and fibrosis. American Journal of Physiology. Lung Cellular and Molecular Physiology 302 (2): L193-205.
Wesslau, K.P., A. Stein, M. Kasper, and K. Barth. 2019. P2X7 receptor indirectly regulates the JAM-A protein content via modulation of GSK-3beta. International Journal of Molecular Sciences 20 (9): 2298.
Weber, B., M.R. Mendler, I. Lackner, A. von Zelewski, S. Höfler, M. Baur, C.K. Braun, H. Hummler, S. Schwarz, J. Pressmar, and M. Kalbitz. 2019. Lung injury after asphyxia and hemorrhagic shock in newborn piglets: Analysis of structural and inflammatory changes. PLoS One 14 (7): e0219211.
Liao, W.I., S.Y. Wu, S.H. Tsai, H.P. Pao, K.L. Huang, and S.J. Chu. 2021. 2-Methoxyestradiol protects against lung ischemia/reperfusion injury by upregulating annexin A1 protein expression. Frontiers in Immunology 12: 596376.
Haihua, C., W. Wei, H. Kun, L. Yuanli, and L. Fei. 2018. Cobra venom factor-induced complement depletion protects against lung ischemia reperfusion injury through alleviating blood-air barrier damage. Scientific Reports 8 (1): 10346.
**ao, M.M., C.S. Pan, Y.Y. Liu, L.Q. Ma, L. Yan, J.Y. Fan, C.S. Wang, R. Huang, and J.Y. Han. 2017. Post-treatment with Ma-Huang-Tang ameliorates cold-warm-cycles induced rat lung injury. Scientific Reports 7 (1): 312.
Imtiazul, I.M., R. Asma, J.H. Lee, N.J. Cho, S. Park, H.Y. Song, and H.W. Gil. 2019. Change of surfactant protein D and A after renal ischemia reperfusion injury. PLoS One 14 (12): e0227097.
Zhou, J., H. Lian, G. Xu, and T. Zhao. 2020. MicroRNA-451 increases vascular permeability and suppresses angiogenesis in pulmonary burn injury in a rat model. Advances in Clinical Experimental Medicine: Official Organ Wroclaw Medical University 29 (11): 1241–1248.
Liu, X., J. Yang, J. Li, C. Xu, and W. Jiang. 2021. Vanillin attenuates cadmium-induced lung injury through inhibition of inflammation and lung barrier dysfunction through activating AhR. Inflammation 44 (6): 2193–2202.
Xu, X., Q. Zhu, R. Zhang, Y. Wang, F. Niu, W. Wang, D. Sun, and A. Wang. 2017. ITRAQ-based proteomics analysis of acute lung injury induced by oleic acid in mice. Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology 44 (5): 1949–1964.
Geng, P., F. Yu, D. Tan, J. Xu, Y. Yang, M. Xu, H. Wang, and B. Ling. 2020. Involvement of claudin-5 in H2S-induced acute lung injury. The Journal of Toxicological Sciences 45 (5): 293–304.
Filipczak, P.T., A.P. Senft, J. Seagrave, W. Weber, P.J. Kuehl, L.E. Fredenburgh, J.D. McDonald, and R.M. Baron. 2015. NOS-2 inhibition in phosgene-induced acute lung injury. Toxicological Sciences: an Official Journal of the Society of Toxicology 146 (1): 89–100.
Liu, Y., J. Tang, J. Yuan, C. Yao, K. Hosoi, Y. Han, S. Yu, H. Wei, and G. Chen. 2020. Arsenite-induced downregulation of occludin in mouse lungs and BEAS-2B cells via the ROS/ERK/ELK1/MLCK and ROS/p38 MAPK signaling pathways. Toxicology Letters 332: 146–154.
Wang, Y.F., Y.X. Fei, B. Zhao, Q.Y. Yin, J.P. Zhu, G.H. Ren, B.W. Wang, W.R. Fang, and Y.M. Li. 2020. Ma **ng Shi Gan decoction protects against PM2.5-induced lung injury through suppression of epithelial-to-mesenchymal transition (EMT) and epithelial barrier disruption. Evidence-based Complementary and Alternative Medicine 2020: 7176589.
Zhang, Y., L. Zhang, W. Chen, Y. Zhang, X. Wang, Y. Dong, W. Zhang, and X. Lin. 2021. Shp2 regulates PM2.5-induced airway epithelial barrier dysfunction by modulating ERK1/2 signaling pathway. Toxicology Letters 350: 62–70.
Kawkitinarong, K., L. Linz-McGillem, K.G. Birukov, and J.G. Garcia. 2004. Differential regulation of human lung epithelial and endothelial barrier function by thrombin. American Journal of Respiratory Cell and Molecular Biology 31 (5): 517–527.
Joshi, P.C., A. Mehta, W.S. Jabber, X. Fan, and D.M. Guidot. 2009. Zinc deficiency mediates alcohol-induced alveolar epithelial and macrophage dysfunction in rats. American Journal of Respiratory Cell and Molecular Biology 41 (2): 207–216.
Michaudel, C., C. Mackowiak, I. Maillet, L. Fauconnier, C.A. Akdis, M. Sokolowska, A. Dreher, H.T. Tan, V.F. Quesniaux, B. Ryffel, and D. Togbe. 2018. Ozone exposure induces respiratory barrier biphasic injury and inflammation controlled by IL-33. The Journal of Allergy and Clinical Immunology 142 (3): 942–958.
Kim, B.G., P.H. Lee, S.H. Lee, C.S. Park, and A.S. Jang. 2018. Impact of ozone on claudins and tight junctions in the lungs. Environmental Toxicology 33 (7): 798–806.
Tirpude, N.V., A. Sharma, R. Joshi, M. Kumari, and V. Acharya. 2021. Vitex negundo Linn. extract alleviates inflammatory aggravation and lung injury by modulating AMPK/PI3K/Akt/p38-NF-kappaB and TGF-beta/Smad/Bcl2/caspase/LC3 cascade and macrophages activation in murine model of OVA-LPS induced allergic asthma. Journal of Ethnopharmacology 271: 113894.
Schmit, T., S. Ghosh, R.K. Mathur, T. Barnhardt, G. Ambigapathy, M. Wu, C. Combs, and M.N. Khan. 2020. IL-6 deficiency exacerbates allergic asthma and abrogates the protective effect of allergic inflammation against Streptococcus pneumoniae pathogenesis. The Journal of Immunology 205 (2): 469–479.
Armstrong, S.M., C. Wang, J. Tigdi, X. Si, C. Dumpit, S. Charles, A. Gamage, T.J. Moraes, and W.L. Lee. 2012. Influenza infects lung microvascular endothelium leading to microvascular leak: Role of apoptosis and claudin-5. PLoS One 7 (10): e47323.
Yeh, C.L., L.H. Su, J.M. Wu, P.J. Yang, P.C. Lee, P.D. Chen, C.C. Huang, D.Y. Hsieh, H.J. Wang, S.L. Yeh, and M.T. Lin. 2020. Effects of the glutamine administration on T helper cell regulation and inflammatory response in obese mice complicated with polymicrobial sepsis. Mediators of Inflammation 2020: 8869017.
Smallcombe, C.C., D.T. Linfield, T.J. Harford, V. Bokun, A.I. Ivanov, G. Piedimonte, and F. Rezaee. 2019. Disruption of the airway epithelial barrier in a murine model of respiratory syncytial virus infection. American Journal of Physiology. Lung Cellular and Molecular Physiology 316 (2): L358–L368.
Wang, N., X. Yang, J. Sun, Z. Sun, Q. Ma, Z. Wang, Z. Chen, Z. Wang, F. Hu, H. Wang, L. Zhou, M. Zhang, and J. Xu. 2019. Neutrophil extracellular traps induced by VP1 contribute to pulmonary edema during EV71 infection. Cell Death Discovery 5: 111.
Li, H., S. Singh, R. Potula, Y. Persidsky, and G.D. Kanmogne. 2014. Dysregulation of claudin-5 in HIV-induced interstitial pneumonitis and lung vascular injury. Protective role of peroxisome proliferator-activated receptor-gamma. American Journal of Respiratory and Critical Care Medicine 190(1): 85–97.
Rokkam, D., M.J. Lafemina, J.W. Lee, M.A. Matthay, and J.A. Frank. 2011. Claudin-4 levels are associated with intact alveolar fluid clearance in human lungs. American Journal of Pathology 179 (3): 1081–1087.
Wang, L., R. Bittman, J.G. Garcia, and S.M. Dudek. 2015. Junctional complex and focal adhesion rearrangement mediates pulmonary endothelial barrier enhancement by FTY720 S-phosphonate. Microvascular Research 99: 102–109.
Ohmura, T., Y. Tian, N. Sarich, Y. Ke, A. Meliton, A.S. Shah, K. Andreasson, K.G. Birukov, and A.A. Birukova. 2017. Regulation of lung endothelial permeability and inflammatory responses by prostaglandin A2: Role of EP4 receptor. Molecular Biology of the Cell 28 (12): 1622–1635.
Tian, X., Y. Tian, G. Gawlak, F. Meng, Y. Kawasaki, T. Akiyama, and A.A. Birukova. 2015. Asef controls vascular endothelial permeability and barrier recovery in the lung. Molecular Biology of the Cell 26 (4): 636–650.
Sim, T.Y., H.H. Harith, C.L. Tham, N.F. Md Hashim, K. Shaari, M.R. Sulaiman, and D.A. Israf. 2018. The protective effects of a synthetic geranyl acetophenone in a cellular model of TNF-alpha-induced pulmonary epithelial barrier dysfunction. Molecules 23 (6): 1355.
Gan, T., Y. Yang, F. Hu, X. Chen, J. Zhou, Y. Li, Y. Xu, H. Wang, Y. Chen, and M. Zhang. 2018. TLR3 regulated Poly I:C-induced neutrophil extracellular traps and acute lung injury partly through p38 MAP kinase. Frontiers in Microbiology 9: 3174.
Hu, S., J. Park, A. Liu, J. Lee, X. Zhang, Q. Hao, and J.W. Lee. 2018. Mesenchymal stem cell microvesicles restore protein permeability across primary cultures of injured human lung microvascular endothelial cells. Stem Cells Translational Medicine 7 (8): 615–624.
Overgaard, C.E., B. Schlingmann, S. Dorsainvil White, C. Ward, X. Fan, S. Swarnakar, L.A. Brown, D.M. Guidot, and M. Koval. 2015. The relative balance of GM-CSF and TGF-beta1 regulates lung epithelial barrier function. American Journal of Physiology. Lung Cellular and Molecular Physiology 308 (12): L1212-1223.
Hsiao, H.M., R. Fernandez, S. Tanaka, W. Li, J.H. Spahn, S. Chiu, M. Akbarpour, D. Ruiz-Perez, Q. Wu, C. Turam, D. Scozzi, T. Takahashi, H.P. Luehmann, V. Puri, G.R.S. Budinger, A.S. Krupnick, A.V. Misharin, K.J. Lavine, Y. Liu, A.E. Gelman, A. Bharat, and D. Kreisel. 2018. Spleen-derived classical monocytes mediate lung ischemia-reperfusion injury through IL-1beta. The Journal of Clinical Investigation 128 (7): 2833–2847.
Xu, S., Q. Yang, J. Bai, T. Tao, L. Tang, Y. Chen, C.S. Chung, E.A. Fallon, and A. Ayala. 2020. Blockade of endothelial, but not epithelial, cell expression of PD-L1 following severe shock attenuates the development of indirect acute lung injury in mice. American Journal of Physiology. Lung Cellular and Molecular Physiology 318 (4): L801–L812.
van der Heijden, M., G.P. van Nieuw Amerongen, J. van Bezu, M.A. Paul, A.B. Groeneveld, and V.W. van Hinsbergh. 2011. Opposing effects of the angiopoietins on the thrombin-induced permeability of human pulmonary microvascular endothelial cells. PLoS One 6 (8): e23448.
ACKNOWLEDGEMENTS
We thank Kathy Kyler, Staff Editor at the OUHSC for her editorial help.
Funding
This work was supported by the funding from National Heart, Lung, and Blood Institute of the National Institute of Health under award number R01 HL136325. Dr. Nachiket M. Godbole’s (postdoctoral fellow) stipend was supported through the American Association of Immunologists Careers in Immunology Fellowship Program.
Author information
Authors and Affiliations
Contributions
SA coordinated the literature search, wrote specific sections, and organized and compiled the review manuscript. NMG and AAC reviewed and wrote specific sections of the manuscript. NMG prepared the figure. NC and AAC compiled the tables.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
All authors have contributed to this manuscript, have read, and given their consent for its publication.
Competing Interests
The authors declare no competing interests.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health or the American Association of Immunologists.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Godbole, N.M., Chowdhury, A.A., Chataut, N. et al. Tight Junctions, the Epithelial Barrier, and Toll-like Receptor-4 During Lung Injury. Inflammation 45, 2142–2162 (2022). https://doi.org/10.1007/s10753-022-01708-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-022-01708-y