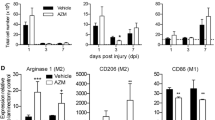
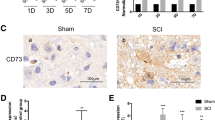
ABSTRACT
Macrophage activation and persistent inflammation contribute to the pathogenesis of spinal cord injury (SCI), and different phenotypes of macrophages play diverse roles in the pathological process of SCI. After SCI, there is an acute phase of alternatively activated (M2) macrophage infiltration, followed by a long-lasting phase of classically activated (M1) macrophage accumulation in the wound. The long-lasting predominance of M1 macrophages may derail healing and compromise organ functions. Based on the previous findings that the transcription factor interferon regulatory factor 5 (IRF5) up-regulates genes associated with M1 macrophages, we attempted to examine the effect of silencing IRF5 on SCI progression. IRF5 expression was assessed with Western blotting or immunohistochemistry. Macrophage phenotypes were measured with flow cytometry or immunohistochemistry. M1- or M2-related cytokines were measured with a Luminex assay kit. IRF5 siRNA was delivered into the macrophages infiltrated into the wound of SCI mice through lipidoid nanoparticle. Locomotor functions were measured with Basso Mouse Scale (BMS) scoring. Myelination was assessed with luxol fast blue staining. Myelin binding protein, neurofilaments, synaptic markers, and cytokines in the wound area were measured with Western blotting. The Mann–Whitney U test was used for statistical analyses. After SCI, significant elevation of IRF5 was evident on day 1, peaked on day 7, and gradually decreased thereafter. Similar dynamic change in the expression of CD86, a typical M1 marker, was observed. In contrast, there was a transient increase in the expression of CD206, a typical M2 marker, which peaked 6 h after SCI, and returned to baseline within 1 day. Macrophages isolated from the epicenter at day 3 after SCI were predominantly M1 phenotype, and a siRNA-mediated knockdown of IRF5 resulted in a reduced expression of M1 macrophage markers and increased expression of M2 macrophage markers. Nanoparticle-mediated delivery of IRF5 siRNA to SCI mouse model resulted in a dramatic decrease in the number of M1 macrophages and a significant increase in the number of M2 macrophages in the wound. This was associated with a robust inflammation resolution, attenuation of demyelination and neurofilament loss, and significant improvement of locomotor function (p < 0.05). IRF5 may serve as a therapeutic target to promote post-SCI recovery.






Similar content being viewed by others
References
Akinc, A., A. Zumbuehl, M. Goldberg, E.S. Leshchiner, V. Busini, N. Hossain, S.A. Bacallado, D.N. Nguyen, J. Fuller, R. Alvarez, et al. 2008. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nature Biotechnology 26: 561–569.
Alabi, C., A. Vegas, and D. Anderson. 2012. Attacking the genome: emerging siRNA nanocarriers from concept to clinic. Current Opinion in Pharmacology 12: 427–433.
Aouadi, M., G.J. Tesz, S.M. Nicoloro, M. Wang, M. Chouinard, E. Soto, G.R. Ostroff, and M.P. Czech. 2009. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature 458: 1180–1184.
Basso, D.M., L.C. Fisher, A.J. Anderson, L.B. Jakeman, D.M. McTigue, and P.G. Popovich. 2006. Basso mouse scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. Journal of Neurotrauma 23: 635–659.
Blight, A.R. 2002. Miracles and molecules—progress in spinal cord repair. Nature Neuroscience 5(Suppl): 1051–1054.
Bomstein, Y., J.B. Marder, K. Vitner, I. Smirnov, G. Lisaey, O. Butovsky, V. Fulga, and E. Yoles. 2003. Features of skin-coincubated macrophages that promote recovery from spinal cord injury. Journal of Neuroimmunology 142: 10–16.
Busch, S.A., J.A. Hamilton, K.P. Horn, F.X. Cuascut, R. Cutrone, N. Lehman, R.J. Deans, A.E. Ting, R.W. Mays, and J. Silver. 2011. Multipotent adult progenitor cells prevent macrophage-mediated axonal dieback and promote regrowth after spinal cord injury. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience 31: 944–953.
Carlson, S.L., M.E. Parrish, J.E. Springer, K. Doty, and L. Dossett. 1998. Acute inflammatory response in spinal cord following impact injury. Experimental Neurology 151: 77–88.
Chen, K.B., K. Uchida, H. Nakajima, T. Yayama, T. Hirai, S. Watanabe, A.R. Guerrero, S. Kobayashi, W.Y. Ma, S.Y. Liu, and H. Baba. 2011. Tumor necrosis factor-alpha antagonist reduces apoptosis of neurons and oligodendroglia in rat spinal cord injury. Spine 36: 1350–1358.
Courties, G., T. Heidt, M. Sebas, Y. Iwamoto, D. Jeon, J. Truelove, B. Tricot, G. Wojtkiewicz, P. Dutta, H.B. Sager, et al. 2014. In vivo silencing of the transcription factor IRF5 reprograms the macrophage phenotype and improves infarct healing. Journal of the American College of Cardiology 63: 1556–1566.
David, S., A.D. Greenhalgh, and A. Kroner. 2015. Macrophage and microglial plasticity in the injured spinal cord. Neuroscience 307: 311–318.
David, S., and A. Kroner. 2011. Repertoire of microglial and macrophage responses after spinal cord injury. Nature Reviews. Neuroscience 12: 388–399.
Downs, T.R., and W.W. Wilfinger. 1983. Fluorometric quantification of DNA in cells and tissue. Analytical Biochemistry 131: 538–547.
Fleming, J.C., M.D. Norenberg, D.A. Ramsay, G.A. Dekaban, A.E. Marcillo, A.D. Saenz, M. Pasquale-Styles, W.D. Dietrich, and L.C. Weaver. 2006. The cellular inflammatory response in human spinal cords after injury. Brain: a Journal of Neurology 129: 3249–3269.
Gensel, J.C., S. Nakamura, Z. Guan, N. van Rooijen, D.P. Ankeny, and P.G. Popovich. 2009. Macrophages promote axon regeneration with concurrent neurotoxicity. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience 29: 3956–3968.
Giger, R.J., E.R. Hollis 2nd, and M.H. Tuszynski. 2010. Guidance molecules in axon regeneration. Cold Spring Harbor Perspectives in Biology 2: a001867.
Gordon, S., and F.O. Martinez. 2010. Alternative activation of macrophages: mechanism and functions. Immunity 32: 593–604.
Guerrero, A.R., K. Uchida, H. Nakajima, S. Watanabe, M. Nakamura, W.E. Johnson, and H. Baba. 2012. Blockade of interleukin-6 signaling inhibits the classic pathway and promotes an alternative pathway of macrophage activation after spinal cord injury in mice. Journal of Neuroinflammation 9: 40.
Hausmann, O.N. 2003. Post-traumatic inflammation following spinal cord injury. Spinal Cord 41: 369–378.
Horn, K.P., S.A. Busch, A.L. Hawthorne, N. van Rooijen, and J. Silver. 2008. Another barrier to regeneration in the CNS: activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience 28: 9330–9341.
Khayrullina, G., S. Bermudez, and K.R. Byrnes. 2015. Inhibition of NOX2 reduces locomotor impairment, inflammation, and oxidative stress after spinal cord injury. Journal of Neuroinflammation 12: 172.
Kigerl, K.A., J.C. Gensel, D.P. Ankeny, J.K. Alexander, D.J. Donnelly, and P.G. Popovich. 2009. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience 29: 13435–13444.
Krausgruber, T., K. Blazek, T. Smallie, S. Alzabin, H. Lockstone, N. Sahgal, T. Hussell, M. Feldmann, and I.A. Udalova. 2011. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nature Immunology 12: 231–238.
Kroner, A., A.D. Greenhalgh, J.G. Zarruk, R. Passos Dos Santos, M. Gaestel, and S. David. 2014. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron 83: 1098–1116.
Laskin, D.L. 2009. Macrophages and inflammatory mediators in chemical toxicity: a battle of forces. Chemical Research in Toxicology 22: 1376–1385.
Leuschner, F., P. Dutta, R. Gorbatov, T.I. Novobrantseva, J.S. Donahoe, G. Courties, K.M. Lee, J.I. Kim, J.F. Markmann, B. Marinelli, et al. 2011. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nature Biotechnology 29: 1005–1010.
Li, F., B. Cheng, J. Cheng, D. Wang, H. Li, and X. He. 2015. CCR5 blockade promotes M2 macrophage activation and improves locomotor recovery after spinal cord injury in mice. Inflammation 38: 126–133.
Lobatto, M.E., V. Fuster, Z.A. Fayad, and W.J. Mulder. 2011. Perspectives and opportunities for nanomedicine in the management of atherosclerosis. Nature Reviews. Drug Discovery 10: 835–852.
Love, K.T., K.P. Mahon, C.G. Levins, K.A. Whitehead, W. Querbes, J.R. Dorkin, J. Qin, W. Cantley, L.L. Qin, T. Racie, et al. 2010. Lipid-like materials for low-dose, in vivo gene silencing. Proceedings of the National Academy of Sciences of the United States of America 107: 1864–1869.
Ma, S.F., Y.J. Chen, J.X. Zhang, L. Shen, R. Wang, J.S. Zhou, J.G. Hu, and H.Z. Lu. 2015. Adoptive transfer of M2 macrophages promotes locomotor recovery in adult rats after spinal cord injury. Brain, Behavior, and Immunity 45: 157–170.
Mantovani, A., A. Sica, S. Sozzani, P. Allavena, A. Vecchi, and M. Locati. 2004. The chemokine system in diverse forms of macrophage activation and polarization. Trends in Immunology 25: 677–686.
Novobrantseva, T.I., A. Borodovsky, J. Wong, B. Klebanov, M. Zafari, K. Yucius, W. Querbes, P. Ge, V.M. Ruda, S. Milstein, et al. 2012. Systemic RNAi-mediated gene silencing in nonhuman primate and rodent myeloid cells. Molecular Therapy Nucleic Acids 1: e4.
Novrup, H.G., V. Bracchi-Ricard, D.G. Ellman, J. Ricard, A. Jain, E. Runko, L. Lyck, M. Yli-Karjanmaa, D.E. Szymkowski, D.D. Pearse, et al. 2014. Central but not systemic administration of XPro1595 is therapeutic following moderate spinal cord injury in mice. Journal of Neuroinflammation 11: 159.
Papa, S., I. Caron, E. Erba, N. Panini, M. De Paola, A. Mariani, C. Colombo, R. Ferrari, D. Pozzer, E.R. Zanier, et al. 2016. Early modulation of pro-inflammatory microglia by minocycline loaded nanoparticles confers long lasting protection after spinal cord injury. Biomaterials 75: 13–24.
Peer, D., E.J. Park, Y. Morishita, C.V. Carman, and M. Shimaoka. 2008. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science 319: 627–630.
Pineau, I., and S. Lacroix. 2007. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. The Journal of Comparative Neurology 500: 267–285.
Popovich, P.G., Z. Guan, P. Wei, I. Huitinga, N. van Rooijen, and B.T. Stokes. 1999. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Experimental Neurology 158: 351–365.
Rettig, G.R., and M.A. Behlke. 2012. Progress toward in vivo use of siRNAs-II. Molecular Therapy: the Journal of the American Society of Gene Therapy 20: 483–512.
Schwartz, M. 2010. “Tissue-repairing” blood-derived macrophages are essential for healing of the injured spinal cord: from skin-activated macrophages to infiltrating blood-derived cells? Brain, Behavior, and Immunity 24: 1054–1057.
Sun, X., X. Wang, T. Chen, T. Li, K. Cao, A. Lu, Y. Chen, D. Sun, J. Luo, J. Fan, et al. 2010. Myelin activates FAK/Akt/NF-kappaB pathways and provokes CR3-dependent inflammatory response in murine system. PLoS One 5: e9380.
Takaoka, A., H. Yanai, S. Kondo, G. Duncan, H. Negishi, T. Mizutani, S. Kano, K. Honda, Y. Ohba, T.W. Mak, and T. Taniguchi. 2005. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature 434: 243–249.
Thompson, C.D., J.C. Zurko, B.F. Hanna, D.J. Hellenbrand, and A. Hanna. 2013. The therapeutic role of interleukin-10 after spinal cord injury. Journal of Neurotrauma 30: 1311–1324.
Weiss, M., K. Blazek, A.J. Byrne, D.P. Perocheau, and I.A. Udalova. 2013. IRF5 is a specific marker of inflammatory macrophages in vivo. Mediators of Inflammation 2013: 245804.
Whitehead, K.A., R. Langer, and D.G. Anderson. 2009. Knocking down barriers: advances in siRNA delivery. Nature Reviews. Drug Discovery 8: 129–138.
Acknowledgments
This research was supported by grants from the National Natural Science Foundation of China (No. 31371011)
Authors’ Contributions
Qiang Fu oversaw the whole project, designed the experiments, and provided scientific input; Jun Li performed experiments for Figs. 1, 2, and 3 and completed the manuscript; Yanbin Liu performed studies for Figs. 4 and 5; and Haidong Xu performed studies for Fig. 6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures were performed in accordance with the Changhai Hospital Institutional Animal Care and Use Committee.
Conflict of Interest
The authors declare that they have no conflict of interests.
Additional information
Jun Li and Yanbin Liu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, J., Liu, Y., Xu, H. et al. Nanoparticle-Delivered IRF5 siRNA Facilitates M1 to M2 Transition, Reduces Demyelination and Neurofilament Loss, and Promotes Functional Recovery After Spinal Cord Injury in Mice. Inflammation 39, 1704–1717 (2016). https://doi.org/10.1007/s10753-016-0405-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-016-0405-4