ABSTRACT
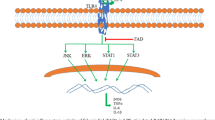
We isolated JEUD-38, a new sesquiterpene lactone from Inula japonica Thunb. JEUD-38 dramatically attenuated lipopolysaccharide (LPS)-induced nitric oxide (NO) production. Consistent with this finding, the protein expression of inducible nitric oxide synthase (iNOS) was blocked by JEUD-38 in a concentration-dependent manner. To elucidate the mechanism, we examined the effect of JEUD-38 on LPS-stimulated nuclear factor-κB (NF-κB) nuclear translocation, inhibitory factor-κB (IκB) phosphorylation, and degradation. JEUD-38 reduced the translocation of p65, via abrogating IκB-α phosphorylation and degradation. In addition, JEUD-38 inhibited LPS-stimulated phosphorylation of mitogen-activated protein kinases (MAPKs) including extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK), and p38. Since iNOS as well as the upstream NF-κB and MAPKs are known to be closely involved in inflammation, these results suggest that JEUD-38 is a promising candidate for prevention and therapy of inflammatory diseases.






Similar content being viewed by others
REFERENCES
Su, X., and H.J. Federoff. 2014. Immune responses in Parkinson's disease: Interplay between central and peripheral immune systems. BioMed Research International 275178.
Virdis, A., U. Dell'Agnello, and S. Taddei. 2014. Impact of inflammation on vascular disease in hypertension. Maturitas 78: 179–183.
Gordon, S. 2003. Alternative activation of macrophages. Nature Reviews. Immunology 3: 23–35.
Rim, H.K., W. Cho, S.H. Sung, and K.T. Lee. 2012. Nodakenin suppresses lipopolysaccharide-induced inflammatory responses in macrophage cells by inhibiting tumor necrosis factor receptor-associated factor 6 and nuclear factor-kappaB pathways and protects mice from lethal endotoxin shock. Journal of Pharmacology and Experimental Therapeutics 342: 654–664.
Wimalawansa, S.J. 2008. Nitric oxide: New evidence for novel therapeutic indications. Expert Opinion on Pharmacotherapy 9: 1935–1954.
Chesrown, S.E., J. Monnier, G. Visner, and H.S. Nick. 1994. Regulation of inducible nitric oxide synthase mRNA levels by LPS, INF-gamma, TGF-beta, and IL-10 in murine macrophage cell lines and rat peritoneal macrophages. Biochemical and Biophysical Research Communications 200: 126–134.
Bogdan, C., M. Rollinghoff, and A. Diefenbach. 2000. The role of nitric oxide in innate immunity. Immunological Reviews 173: 17–26.
MacMicking, J., Q.W. **e, and C. Nathan. 1997. Nitric oxide and macrophage function. Annual Review of Immunology 15: 323–350.
Sharma, J.N., A. Al-Omran, and S.S. Parvathy. 2007. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 15: 252–259.
Barnes, P.J., and M. Karin. 1997. Nuclear factor-kappaB: A pivotal transcription factor in chronic inflammatory diseases. New England Journal of Medicine 336: 1066–1071.
Chen, F., V. Castranova, X. Shi, and L.M. Demers. 1999. New insights into the role of nuclear factor-kappaB, a ubiquitous transcription factor in the initiation of diseases. Clinical Chemistry 45: 7–17.
Baldwin Jr., A.S. 2001. Series introduction: the transcription factor NF-kappaB and human disease. Journal of Clinical Investigation 107: 3–6.
Luo, J.L., H. Kamata, and M. Karin. 2005. IKK/NF-kappaB signaling: Balancing life and death—a new approach to cancer therapy. Journal of Clinical Investigation 115: 2625–2632.
Brown, M.D., and D.B. Sacks. 2008. Compartmentalised MAPK pathways. Handbook of Experimental Pharmacology 205–235.
Chan, E.D., and D.W. Riches. 1998. Potential role of the JNK/SAPK signal transduction pathway in the induction of iNOS by TNF-alpha. Biochemical and Biophysical Research Communications 253: 790–796.
Ajizian, S.J., B.K. English, and E.A. Meals. 1999. Specific inhibitors of p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways block inducible nitric oxide synthase and tumor necrosis factor accumulation in murine macrophages stimulated with lipopolysaccharide and interferon-gamma. Journal of Infectious Diseases 179: 939–944.
Yang, C., C.M. Wang, and Z.J. Jia. 2003. Sesquiterpenes and other constituents from the aerial parts of Inula japonica. Planta Medica 69: 662–666.
Park, Y.N., Y.J. Lee, J.H. Choi, M. **, J.H. Yang, Y. Li, J. Lee, X. Li, K.J. Kim, J.K. Son, H.W. Chang, J.Y. Kim, and E. Lee. 2011. Alleviation of OVA-induced airway inflammation by flowers of Inula japonica in a murine model of asthma. Bioscience, Biotechnology, and Biochemistry 75: 871–876.
Lu, Y., Y. Li, M. **, J.H. Yang, X. Li, G.H. Chao, H.H. Park, Y.N. Park, J.K. Son, E. Lee, and H.W. Chang. 2012. Inula japonica extract inhibits mast cell-mediated allergic reaction and mast cell activation. Journal of Ethnopharmacology 143: 151–157.
Choi, J.H., Y.N. Park, Y. Li, M.H. **, J. Lee, Y. Lee, J.K. Son, H.W. Chang, and E. Lee. 2010. Flowers of Inula japonica attenuate inflammatory responses. Immune Network 10: 145–152.
**, M., Q. Zhou, E. Lee, S. Dan, H.Q. Duan, and D. Kong. 2014. AS252424, a PI3Kgamma inhibitor, downregulates inflammatory responsiveness in mouse bone marrow-derived mast cells. Inflammation 37: 1254–1260.
Bohlmann, F., P.K. Mahanta, J. Jakupovic, R.C. Rastogi, and A.A. Natu. 1978. New sesquiterpene lactones from Inula species. Phytochemistry 17: 1165–1172.
An, H.J., I.T. Kim, H.J. Park, H.M. Kim, J.H. Choi, and K.T. Lee. 2011. Tormentic acid, a triterpenoid saponin, isolated from Rosa rugosa, inhibited LPS-induced iNOS, COX-2, and TNF-alpha expression through inactivation of the nuclear factor-kappab pathway in RAW 264.7 macrophages. International Immunopharmacology 11: 504–510.
Lindstrom, T.M., and P.R. Bennett. 2005. The role of nuclear factor kappa B in human labour. Reproduction 130: 569–581.
Guha, M., and N. Mackman. 2001. LPS induction of gene expression in human monocytes. Cellular Signalling 13: 85–94.
Chan, E.D., and D.W. Riches. 2001. IFN-gamma + LPS induction of iNOS is modulated by ERK, JNK/SAPK, and p38 (mapk) in a mouse macrophage cell line. American Journal of Physiology. Cell Physiology 280: C441–C450.
**, M., S.J. Suh, J.H. Yang, Y. Lu, S.J. Kim, S. Kwon, T.H. Jo, J.W. Kim, Y.I. Park, G.W. Ahn, C.K. Lee, C.H. Kim, J.K. Son, K.H. Son, and H.W. Chang. 2010. Anti-inflammatory activity of bark of Dioscorea batatas DECNE through the inhibition of iNOS and COX-2 expressions in RAW264.7 cells via NF-kappaB and ERK1/2 inactivation. Food and Chemical Toxicology 48: 3073–3079.
Alderton, W.K., C.E. Cooper, and R.G. Knowles. 2001. Nitric oxide synthases: Structure, function and inhibition. Biochemical Journal 357: 593–615.
Andrew, P.J., and B. Mayer. 1999. Enzymatic function of nitric oxide synthases. Cardiovascular Research 43: 521–531.
Sanghera, J.S., S.L. Weinstein, M. Aluwalia, J. Girn, and S.L. Pelech. 1996. Activation of multiple proline-directed kinases by bacterial lipopolysaccharide in murine macrophages. Journal of Immunology 156: 4457–4465.
He, X., Z. **g, and G. Cheng. 2014. MicroRNAs: New regulators of Toll-like receptor signalling pathways. BioMed Research International 945169.
Sun, X.F., and H. Zhang. 2007. NFKB and NFKBI polymorphisms in relation to susceptibility of tumour and other diseases. Histology and Histopathology 22: 1387–1398.
Baeuerle, P.A., and D. Baltimore. 1996. NF-kappa B: Ten years after. Cell 87: 13–20.
Murakami, A. 2009. Chemoprevention with phytochemicals targeting inducible nitric oxide synthase. Forum of Nutrition 61: 193–203.
Bhat, N.R., P. Zhang, J.C. Lee, and E.L. Hogan. 1998. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. Journal of Neuroscience 18: 1633–1641.
Shan, J.J., M. Yang, and J.W. Ren. 2006. Anti-diabetic and hypolipidemic effects of aqueous-extract from the flower of Inula japonica in alloxan-induced diabetic mice. Biological & Pharmaceutical Bulletin 29: 455–459.
Shan, J.J., Y. Zhang, Y.L. Diao, W.S. Qu, and X.N. Zhao. 2010. Effect of an antidiabetic polysaccharide from Inula japonica on constipation in normal and two models of experimental constipated mice. Phytotherapy Research 24: 1734–1738.
ACKNOWLEDGMENTS
This study was supported by a grant from the National Natural Science Foundation of China (81373441, 81202542, 81402802), the Natural Science Foundation of Tian** (12JCZDJC25800, 13JCYBJC24800), the New Teachers’ Fund for Doctor Stations from Ministry of Education, China (20121202120009), the Project Sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry, a grant from “211” project of Tian** Medical University, and a grant from Japan Society for the Promotion of Science (FY2013, BR131302).
Conflict of Interest
There is no conflict of interest for all authors of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, X., Tang, SA., Wang, R. et al. Inhibitory Effects of JEUD-38, a New Sesquiterpene Lactone from Inula japonica Thunb, on LPS-Induced iNOS Expression in RAW264.7 Cells. Inflammation 38, 941–948 (2015). https://doi.org/10.1007/s10753-014-0056-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-014-0056-2