Abstract
The parasitic copepod Neoergasilus japonicus (Ergasilidae), native to East Asia, has spread rapidly around the world over the past half-century and now represents a clear example of successful parasitic invader. The species is now found in western Asia, Europe, America and Africa, with aquaculture and fish introductions identified as the primary vectors of dispersal. Regional field investigations have revealed surprisingly high number of affected localities, indicating potentially wider distribution than currently recognised. Neoergasilus japonicus exhibits low host specificity, parasitising a diverse range of freshwater fishes. This study updates the global fish host species list to 132, spanning 27 families across 15 orders, with Cypriniformes identified as the most susceptible host species. Under experimental conditions in this study, however, N. japonicus avoided its natural host, topmouth gudgeon Pseudorasbora parva, suggesting a level of resistance to its native parasite. Piscivorous and demersal fish were less infected by copepods than planktivorous, benthivorous, pelagic and benthopelagic species, reflecting the ecology of both the parasite and its hosts. An ability to re-attach to another host, though limited, was confirmed under experimental conditions. Recognising the ecological impacts and potential consequences associated with the introduction of non-native parasites emphasises the need for continuous monitoring and research globally.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The introduction of parasites alongside their hosts is quite common, though their numbers and species richness often diminish in the new range compared to their original range (Torchin et al., 2003). Registering these parasites typically takes longer than discovering their hosts due to their smaller size and lack of immediate visibility. Moreover, not all invasive species undergo parasite screening immediately upon detection. Indeed, many co-introduced parasites only become noticeable when they switch to native hosts (Lymbery et al., 2014). However, an increasing number of studies now also show co-introduced parasites with their non-indigenous vectors (e.g. Kmentová et al., 2019; Šimková et al., 2019; Ondračková et al., 2023).
Parasite survival following its introduction and subsequent establishment will depend on a range of factors, the most important being introduction of a sufficiently large founder population (Sakai et al., 2001), tolerance of the parasite or its developmental stages to the new environmental conditions (MacLeod et al., 2010) and the presence of all components (hosts) of the parasite’s life cycle (Hoberg, 2010). Parasite specificity plays an important role in determining whether a co-introduced parasite remains confined to its natural non-indigenous host in a new geographic area or is able to switch to native species (Poulin et al., 2011). In a meta-analysis encompassing almost one hundred studies on parasite co-introductions, Lymbery et al. (2014) revealed that 78% of co-introduced parasites switched from their natural hosts to a local native host. In some cases, co-introduced pathogens may then exhibit increased virulence (i.e. an enhanced ability to cause disease) in the new host due to a lack of innate immunity compared with their natural host (Peeler et al., 2011).
Using the definition of Kolar & Lodge (2001), invasive species are considered non-indigenous organisms introduced outside their natural range, establishing self-sustaining populations, amplifying, expanding and causing deleterious impacts on the environment, economy or human health. Both free-living and parasitic organisms may become invasive (Taraschewski, 2006). Among the parasite species known to have been co-introduced with their hosts into new areas, only a few have managed to spread to a wide range of local hosts and become invasive. One example of such a successful invasive freshwater parasite is the Asian fish tapeworm Schyzocotyle acheilognathi (Yamaguti, 1934). This parasite has spread to almost all continents, infecting 312 fish species and 11 non-fish species (Kuchta et al., 2018). Another example is the widespread crustacean parasite Lernaea cyprinacea Linnaeus, 1758, recognised as one of the most invasive ectoparasites of freshwater fish (Welicky et al., 2017). Interestingly, in affected areas, native fish species tend to be more vulnerable to this parasite (Waicheim et al., 2019). Understanding the timelines and host associations of invasion and colonisation aids in anticipating the speed and success of future colonisation events by invasive parasites (Kuchta et al., 2018); nevertheless, studies summarising the geographical distribution and host range of such parasites are relatively scarce.
Neoergasilus japonicus (Harada, 1930) is an ergasilid copepod, native to East Asia (Kim & Choi, 2003). It was originally described as Ergasilus japonicus Harada, 1930 from cyprinid fishes (e.g. topmouth gudgeon Pseudorasbora parva (Temminck & Schlegel, 1846)) in Taiwan (Harada, 1930), and later transferred to the genus Neoergasilus by Yin (1956). As in other ergasilids, only fertilised females are parasitic, while males are free-living (Urawa et al., 1980). Co-introduced with its fish hosts, the species has adapted to local fish species, spreading across a broad geographical range and a range of host species, and establishing abundant populations in some regions (Hudson & Bowen, 2002). Consequently, it has been denoted as a very high-priority species for risk assessment in some localities, including the Great Lakes in the USA (Bell et al., 2023), supporting N. japonicus to be classified as an invasive parasite species. There is evidence suggesting that N. japonicus was introduced to Europe with ornamental fish imported from East Asia (Alekseev et al., 2021). In Europe, the parasite was initially recorded in Hungary and Slovakia in the 1960s (Hanek, 1968; Pónyi & Molnár, 1969). Subsequently, it was reported in Cuba in the 1980s (Prieto et al., 1985), and in continental America in the 1990s (Hayden & Rogers, 1998; Suárez-Morales & Mercado-Salas, 2013). The recent expansion of its geographical range has been attributed to a rapid increase in fish introductions associated with global aquacultural activities (Suárez-Morales & Mercado-Salas, 2013). Currently, N. japonicus is widely distributed in Asia, Europe and America, with reports also emerging from several countries in Africa (Nagasawa & Uyeno, 2012; references in Online Resource 1). The pathogenicity of N. japonicus to fish hosts appears relatively low compared to other parasitic crustaceans, possibly due in part to its smaller size or its location on the skin (Avenant-Oldewage et al., 2023). Nevertheless, fish host tissue has been observed in the parasite’s intestine, indicating that parasitic adult females consume the host epidermis, causing local trauma (Avenant-Oldewage et al., 2023). In addition, there are instances reported in China, where N. japonicus has caused the death of crucian carp Carassius carassius (Linnaeus, 1758) and chub Squalius cephalus (Linnaeus, 1758) (Gao et al., 2010), and in Great Britain, where it slowed the growth of juvenile roach Rutilus rutilus (Linnaeus, 1758) (Mugridge et al., 1982). Furthermore, the presence of free-living stages of the parasite (i.e. copepodite stages and males) in low-nutrient waters has been shown to affect local plankton communities (Alekseev et al., 2021). Copepodite (larval) stages are predators of small zooplanktonic organisms and can exert significant predation pressure, particularly in autumn, when the last generation reproduces before hibernation. Copepodites in one French glacial lake, for example, caused a 25% reduction in the population of native rotifers and free-living arthropod larvae in the surface water zones, despite constituting only 1% of the total zooplankton biomass (Alekseev et al., 2021). Interestingly, Velásquez-Ornelas et al. (2021) reported that N. japonicus parasitised even copepods Mesocylops edax (Forbes, 1891).
Reports of N. japonicus infecting a diverse range of teleost fishes worldwide, as summarised in Nagasawa & Uyeno (2012), confirm its ability to survive on a range of host fish species, including protected species (Suárez-Morales et al., 2010). Despite its broad host range, certain fish species or families do seem to be reported as hosts more frequently or exhibit higher infection intensities. Among the Cyprinidae, for example, over 50 species have been reported for N. japonicus (Nagasawa & Uyeno, 2012), with rudd Scardinius erythrophthalmus (Linnaeus, 1758) showing particularly high susceptibility to infection in Europe (Kus & Soylu, 2013; Kvach et al., 2021). High prevalence and abundance have also been observed in centrarchid fishes, such as bluegill Lepomis macrochirus Rafinesque, 1819 introduced to Japan (Nagasawa & Sato, 2015) and pumpkinseed Lepomis gibbosus (Linnaeus, 1758) in its native range (Hudson & Bowen, 2002) and in populations introduced to the Czech Republic (Ondračková et al., 2019) and France (Alekseev et al., 2021). However, most of the studies provide “case” reports of selected fish species, often neglecting the broader fish community. Previous research has also indicated that gravid (non-ovigerous) females of N. japonicus can swim and transfer from one fish to another, detaching and re-attaching to another host (Pónyi & Molnár, 1969). While ovigerous females were thought to be unable to switch hosts once attached (Alfonso & Belmonte, 2010), our own examination in Petri dishes has revealed detached ovigerous females swimming in the water, suggesting that females with developed ovary sacs can also detach from host fish tissue, swim freely and potentially re-attach to fish tissue once again. This ability, if it also works in the natural environment, could further contribute to the parasite’s survival in a new environment, highlighting its potential as an additional trait supporting invasive success.
The main objective of this study is to summarise available data on the historical and recent geographic and host distribution of N. japonicus in its native and non-native ranges, based on previously published literature and original field and experimental data. To assess the recent expansion of N. japonicus, we also provide updated lists detailing its recent geographical distribution and all known fish host species. We also set out to determine whether N. japonicus exhibits a preference for some fish taxa, or their ecological traits, by analysing infection levels under both natural and experimental conditions. The combination of both approaches will help resolve whether infection of a particular host taxa is driven by phylogenetic or ecological similarity. Finally, we aim to experimentally test for re-attachment success in ovigerous females, potentially contributing to the parasite’s survival in a new environment.
Materials and methods
To present the recent global distribution and host range of N. japonicus, we conducted a comprehensive review of published data and complemented the information gained with original data collected during our own field surveys (Table 1). Our research employed both field and experimental approaches to evaluate the host preference of N. japonicus at a local scale, specifically in the Czech Republic, Central Europe. The combination of both approaches allowed us to establish a robust understanding of the spatial distribution of N. japonicus and its host preferences within the broader fish host community across diverse aquatic habitats.
Literature survey
First, we gathered a list of records, including location and year of parasite detection or year of publication when the sampling year was unavailable. Additionally, we included information on fish host species infected, when available, as some studies previously confirmed N. japonicus only in zooplankton. We undertook a review of both peer-reviewed journals and grey literature, including scientific reports, conference contributions and BSc., MSc. and PhD theses. We also accessed information from the Web of Knowledge, Google Scholar and the WoRMS databases, using “Neoergasilus japonicus” as a search term. All 344 references in Google Scholar and 32 in Web of Knowledge were analysed and only references providing information on locality (or at least country) where the species was collected, and, where possible, the host species and year of investigation, were selected for further analyses. The chronological records of N. japonicus findings worldwide and a list of fish species in which the parasite has been documented are presented in Online Resource 1 and summarised in Fig. 1.
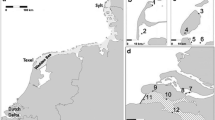
Map indicating occurrence of Neoergasilus japonicus in its expected native (green) and non-native (orange) range (A), with a more detailed view of Europe (B). Year indicates first published record of parasite occurrence in the country. Russia is denoted as both native and non-native range as the parasite naturally occurs in Far East Russia but has been introduced to the western part of the country. India is denoted as a non-native region based on the suggestion of Kumari et al. (2009)
Field sampling
Distribution of N. japonicus in the Czech Republic was assessed based on data collected over 2013–2023. This included published data from our previous research including the first record of the parasite in the country in 2013 (Ondračková et al., 2019, 2021; Kvach et al., 2021) and unpublished personal observations obtained during our routine fieldwork. The dataset encompasses information gathered from 30 lentic water bodies, including aquaculture ponds, sandpits, oxbows, a revitalisation pool and borrow pits (Table 1). It is important to note that this dataset consists of individual records on target fish species and typically not all fish in the sampled locality were examined for presence of the parasite.
To assess the distribution of N. japonicus within the host fish community and evaluate potential host preferences (regarding host ecological traits), we sampled representative fish fauna at five localities during two sampling events in 2023 (see Table 2 for locality details). Fish were collected using electrofishing or beach seining, depending on habitat conditions. In total, fish community composition at the sample sites ranged between 10 and 13 species. All fish caught were quickly anaesthetised by clove oil and examined for the presence of N. japonicus under a stereoscopic microscope. Data recorded included number of parasites and their locality on the fish body, such as fins/fin base, body surface, nose or anal opening. The gills were not examined due to the relatively rare occurrence of N. japonicus at this location (Hudson & Bowen, 2002; Ondračková et al., 2019). Post-examination, the fish were allowed to recover and then released back to the water.
Experimental setup
Experimental investigations to assess potential host preference and re-attachment success of ovigerous females were conducted using four fish species: (1) rudd S. erythrophthalmus, a native Central European cyprinid species highly susceptible to N. japonicus (e.g. Kus & Soylu, 2013; Kvach et al., 2021), (2) roach R. rutilus, another native European cyprinid species showing susceptibility to N. japonicus, albeit with apparently lower abundance compared to S. erythrophthalmus (Elsheikha & Beech, 2017; Kvach et al., 2021), (3) pumpkinseed L. gibbosus, a centrarchid fish introduced to Europe from North America, displaying high susceptibility to N. japonicus (Ondračková et al., 2019; Alekseev et al., 2021; Kvach et al., 2021) and (4) topmouth gudgeon P. parva, a cyprinid fish introduced to Europe from East Asia, representing an original host for N. japonicus (Harada, 1930; Wang, 1964; Smirnova, 1971; Kim & Choi, 2003).
Fish for all experiments were collected from various sites in the Morava river basin (Czech Republic) using electrofishing during June 2021. Subsequently, the fish were transported to the Institute of Vertebrate Biology, Czech Academy of Sciences, where they underwent acclimatisation in outdoor fibreglass tubs (1.4 × 1.4 × 0.9 m; filled with approximately 1.200 l of water) for one week before commencing the experiment. Prior to the experiment, ten fish per species were pre-screened to confirm they are not parasitised with copepods. Additionally, S. erythrophthalmus and L. gibbosus, species highly susceptible to N. japonicus infection, were collected as a source of parasites from the U Jezu sandpit (Morava River basin), where high infection rates have been observed in previous years. Notably, genetic analysis has revealed no difference between copepods collected from S. erythrophthalmus or L. gibbosus at this site (Kvach et al., 2021), allowing mixed parasites from both fish hosts to be used for subsequent experimental infection.
Three experimental setups were conducted. The host preference experiments aimed to determine whether N. japonicus demonstrates preference or avoidance towards particular host species under conditions of equal host availability. This investigation was carried out using short- and long-term setups conducted during the European summer months (June–September 2021), corresponding to the reproductive season of the species (Alekseev et al. 2021). In the short-term setup, copepod-free fish were introduced into a tub containing juvenile stages of N. japonicus. Before infection commenced, ten donor fish (S. erythrophthalmus and L. gibbosus) were kept in the tub for ten days under natural temperature conditions (Online Resource 2). During this period, the larvae could release from egg sacs of adult females and develop into infective stages. The density and developmental stage of larval copepods were checked by examining a subsample of water (3 × 1200 ml) under a light microscope. The test tub was then populated with approximately 2,200 developmental stages of N. japonicus, out of which approximately 40 were females in the final (VI) copepodite stage (i.e. adult, according to Urawa et al., 1991) on the day the experiment began. Six individuals of each potential host species, i.e. R. rutilus, S. erythrophthalmus, L. gibbosus and P. parva, were then placed into the tub. The fish were subjected to a natural light and temperature regime throughout and fed with frozen artemia and chironomid larvae. The experiment terminated on the sixth day, at which point all fish were euthanised and dissected under a binocular microscope and checked for presence of copepod parasites.
The second long-term experimental setup observed host-parasite dynamics over the summer months with a short reproduction cycle, using the same host species and environmental conditions as above (i.e. natural light and temperature regime; see Online Resource 2). The fish were uniquely marked with a coloured visible implant elastomer (VIE; Northwest Marine Technology, USA) close to the dorsal fin base. Four experimental fish per species were placed into a tub equipped with a gravel bottom and artificial and natural (Ceratophyllum spp.) plants. Three adult S. erythrophthalmus naturally infected with N. japonicus were then added to each tub, the setup being run in three replicates. The fish were kept in the tubs for 12 weeks from June to September. Each 3 weeks, all fish were controlled for presence of N. japonicus, resulting in four controls for each fish. After the first control (3 weeks), when parasite larvae had been detected in all three tanks, the S. erythrophthalmus serving as a source host were removed from the tub.
In the final setup, we evaluated the re-attachment success of ovigerous females. Parasites were sourced from S. erythrophthalmus, which were first euthanised by severing the cervical spine. The fins were carefully excised, ensuring minimal damage to the parasites situated at the fin base and placed in a Petri dish filled with dechlorinated water. Parasites released from the severed fin were then employed for experimental infection. Experimental fish were prepared by anaesthetising using clove oil, followed by two rinses in clear dechlorinated water. The anaesthetised fish were then positioned in a small plastic box filled with water positioned under a binocular microscope. Three free-swimming N. japonicus specimens were gently introduced onto the dorsal or anal fin of the fish in a small amount of water using a plastic Pasteur pipette. Once the parasite had attached itself, the experimental fish were carefully released into a large plastic box with water and transferred to outdoor tanks, each partitioned by a fine net into four sections. A total of 12 fish per species underwent individual infection, with three specimens allocated to each of the four tubs, i.e. each tub section housed three specimens of a particular fish species. After four days, the experiment was terminated and all fish were euthanised and dissected to determine presence and developmental stage of N. japonicus.
Data analysis
Parasite infection was characterised according to Bush et al. (1997) as prevalence (i.e. the percentage of infected fish), mean abundance (i.e. the mean number of parasites for all fish in the sample) and intensity of infection (i.e. the number of parasites in an infected fish). The effects of fish host ecological traits (water column position, feeding strategy) on the frequency of N. japonicus prevalence were tested using generalised linear mixed models (GLMM, Bernoulli distribution) in the lme4 package (Bates et al., 2015), with fish standard length as covariate and site as a random effect. To determine the effect of a predictor (i.e. ecological trait), likelihood ratio tests (LR tests) were undertaken comparing the models with and without a term using the lmtest package (Zeileis & Hothorn, 2002).
Inter-specific differences in N. japonicus abundance in the short-term experimental host preference setup were tested using a generalised linear model (GLM, Poisson distribution). The effects of fish host species and time on N. japonicus abundance in the long-term experimental setup were tested using GLMM (negative binomial distribution), with fish standard length as covariate and experimental tub and fish individual as random effects. To determine whether there was a significant interaction between the effect of fish species and time in the experiment, LR tests were undertaken comparing the models with and without interaction. As the effect of the interaction proved significant, we conducted further GLMMs to compare N. japonicus abundance between fish species in each time slot separately. Inter-specific differences in re-attachment success of ovigerous females were tested for using GLMM (binomial distribution) with experimental tub as random effect. In each post hoc comparison, the Tukey HSD approach was applied to control for type II errors in multiple post hoc pairwise comparisons using the glht and mcp functions in the multcomp package (Hothorn et al., 2008). All statistical analyses were conducted using R v.4.3.1 (R Core Team, 2021).
Results
Updated host-parasite list and geographical distribution
The literature survey revealed that N. japonicus has been introduced into water bodies across Southern and Western Asia, Europe, North America, Central America, South America and Africa (Fig. 1). To date, there have been no reported occurrences in Australia and Oceania. The list of countries includes six (i.e. China, Japan, Russia, South Korea, Taiwan, Vietnam) where N. japonicus most probably occurs naturally, at least in some regions, and 23 countries where it has been introduced since 1965. Russia is denoted as a country representing both the species’ native range (Russian Far East) and its introduced range (Moscow region, western part of the country; Online Resource 1). The data suggest a notable increase in the reported records of this species over the past decade (Fig. 2), with recent reports including countries in Africa, South America and Central America (Online Resource 1). Currently, N. japonicus has been identified in 132 fish species, spanning 27 families of 15 orders, namely Cypriniformes, Cichliformes, Centrarchiformes, Cyprinodontiformes, Siluriformes and Perciformes, all with five or more infected species; and Anabatiformes, Beloniformes, Esociformes, Characiformes, Gadiformes, Gobiiformes, Mugiliformes, Salmoniformes and Ovalentaria/misc (sensu Betancur-R et al., 2017), all with 1–2 infected species (Fig. 3). Most infected fish species belong to the families Leuciscidae (29 spp.), Xenocyprinidae (17 spp.) and Cyprinidae, Gobionidae and Cichlidae (each 12 spp., Online Resource 1).
Proportion of records of Neoergasilus japonicus by fish order. “Other” includes orders: Anabatiformes, Beloniformes, Characiformes, Esociformes, Gadiformes, Gobiiformes, Mugiliformes, Salmoniformes and Ovalentaria sensu Betancur-R et al. (2017)
Distribution of N. japonicus at a local scale
Occurrence of N. japonicus in the Czech Republic was documented in seven river basins (out of the eleven main river basins in the country), i.e. those of the Rivers Morava, Dyje and Svratka (Black Sea drainage), and the Vltava, Lužnice, Otava and Elbe (North Sea drainage). Between 2013 and 2023, the parasite was reported in 30 lentic waterbodies, with 14 fish species infected, including five non-native species (Table 1).
Detailed examination of the fish communities at the five localities revealed 19 fish species, 11 of which were infected with N. japonicus (Table 2). Fish of the families Siluridae, Anguillidae, Esocidae and Salmonidae were not found to host N. japonicus; however, their sample size was relatively low. The most frequently infected fish were S. erythrophthalmus, L. gibbosus and European bitterling Rhodeus amarus (Bloch, 1782), all of which were infected at every site sampled. Highest intensity of infection was observed in bleak Alburnus alburnus (Linnaeus, 1758) (10.5) and S. erythrophthalmus (7.4) from the Dedava borrow pit.
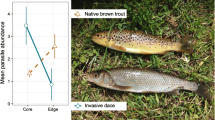
Both fish position in the water column and feeding strategy significantly affected prevalence of N. japonicus (GLMM, LR tests, both P < 0.001). Prevalence in piscivorous fish was significantly lower than in benthivorous and planktivorous fish (GLMM, post hoc tests, both P < 0.001; Fig. 4), and prevalence in demersal fish was significantly lower than in benthopelagic and pelagic fish (GLMM, post hoc tests, P = 0.012 and 0.004, respectively; Fig. 4).
Experimental infection
Females of N. japonicus successfully infected R. rutilus, L. gibbosus and S. erythrophthalmus following short-term (six day) experimental exposure to infective stages, while no parasite was found on P. parva (Table 3). Among the infected fish species, parasite abundance was significantly higher in L. gibbosus than R. rutilus (GLM, P < 0.001; post hoc test, P = 0.049), while other infected species pairs showed no significant difference. Eighteen of 41 observed copepods had developed egg sacs.
Almost no P. parva were infected during long-term experimental exposure, with 48 inspection events resulting in just two records of a single N. japonicus, one at 3 weeks and the other at 6 weeks after the start of the experiment, each in a different experimental tank. Owing to the low variability in P. parva infection, we removed the species from the analysis to prevent computational problems. There were no significant differences in the abundance of N. japonicus between the three remaining fish hosts in the first control (i.e. after 3 weeks; GLMM, LR test, P = 0.950; Fig. 5). Over the next three controls, however, parasite abundance in L. gibbosus significantly exceeded that in both R. rutilus and S. erythrophthalmus (GLMM, LR tests, all P < 0.001; post hoc tests for comparison with S. erythrophthalmus and R. rutilus, respectively: 6 weeks: P = 0.002 and 0.009; 9 weeks: P = 0.013 and 0.009; 12 weeks: P = 0.009 and < 0.001), with no significant differences between the latter two species (post hoc tests = 0.901, 0.564 and 0.999; Fig. 5).
The re-attachment success of ovigerous females was generally very low, with only nine of the 144 (i.e. 6.3%) parasites transferred to experimental fish surviving. All fish species except P. parva remained infected with one or two parasites. While there was no significant difference in N. japonicus survival between host species, the difference was close to significance (GLMM, LR test, P = 0.05).
Discussion
Global distribution
Over the last half-century, the parasitic copepod N. japonicus has spread almost globally. Both historically and more recently, Europe has been the focal point for introduction records. The first published report of N. japonicus outside its native range came from the River Latorica in the Slovak part of the former Czechoslovakia (Hanek, 1968), though the parasite was first observed in Hungary in 1965 (Pónyi & Molnár, 1969). Over the years, the parasite spread to nearby regions in Slovakia, Hungary and Ukraine (Ergens et al., 1975). It remains unclear whether subsequent records in Western Europe relate to this region, or whether the species was introduced into Europe several times. Nevertheless, the parasite was locally reported between 1970 and 2010 from isolated localities in countries such as France (first report in 1978), Great Britain (1980), Finland (1985), Germany (2001) and Italy (2010) (Lescher-Moutoué, 1979; Mugridge et al., 1982; Tuuha et al., 1992; Knopf & Hölker, 2005; Alfonso & Beltmonte, 2010). Over the last decade, the trend shifted to eastern European countries, with findings in the Czech Republic (2013), Bulgaria (2015) and Latvia (2019), as well as Turkey (2012), a transcontinental Euro- Asian country (Soylu & Soylu, 2012; Ondračková et al., 2019, 2021; Kvach et al., 2020). Interestingly, some recent reports highlight higher infection intensities compared to those observed in western countries in the 1970s to 1980s (Ondračková et al., 2019; Alekseev et al., 2021; Kvach et al., 2021). More recently, N. japonicus has been reported in Algeria (Berrouk et al., 2019) and South Africa (Avenant-Oldewage et al., 2023).
While the first record of N. japonicus outside the Eurasian continent was reported in Cuba in 1985 (Prieto et al., 1985), its occurrence on the American continent has been relatively scarce, with reports known from the USA, particularly from the eastern states (Alabama) and around the Great Lakes (Hayden & Rogers, 1998; Hudson & Bowen, 2002; Marshall et al., 2019; Truong & Bullard, 2021). Further records in continental America include Mexico (Orozco Zamorano, 2001) and Peru (Mendes Marques & Morey, 2018). Interestingly, by examining zooplankton samples from the 1990s, Suárez-Morales & Mercado-Salas (2013) found that N. japonicus was already present in continental America (Mexico) even before it was reported in the USA by Hayden & Rogers (1998). This may be a common situation in many other regions. For example, in South Africa, Avenant-Oldewage et al. (2023) noted that the parasite was first reported in 2010, though this finding was only published in conference proceedings, with publicly available information not appearing until 2023. As indicated in most studies reporting a new country or locality record for N. japonicus in non-native regions, it is highly probable that its distribution is much wider as, in many countries, the species is not monitored for in plankton or on fish, or because some records of its occurrence are simply not publicly available. A lack of taxonomic specialists for parasitic copepods may also play an important role. For example, in the recent work by Truter et al. (2023), the authors published a new record of Neoergasilus from African sharptooth catfish Clarias gariepinus (Burchell, 1822) in South Africa, though the parasite was not determined to species level. Furthermore, the parasite’s behaviour, i.e. its ability to release from fish (Pónyi & Molnár, 1969), and its small size may also contribute to its potential oversight during routine fish examination.
Regional distribution in the Czech Republic
The former Czechoslovakia, and particularly the Tisza river basin in the eastern part of Slovakia, was among the first regions where introduced N. japonicus were documented in the 1960s (Hanek, 1968). Since the extensive research on fish parasites in the Tizsa basin by Ergens et al. (1975), however, no further records of N. japonicus were documented from either the Slovak or Czech Republics over the following decades (Moravec, 2001). Occurrence of N. japonicus in the Czech Republic was first documented in two sandpits in the Morava river basin and an oxbow of the River Dyje (Ondračková et al., 2019, 2021). These rivers lie in the Black Sea drainage, as does the River Tisza. Since 2013, the copepod has been recorded at 30 other localities in the Czech Republic, spanning different river basins and drainages of the Black and North Seas (Table 2).
As the Anin and Sever sandpits (see Table 2) in the Morava basin were not investigated for parasitic fauna until 2013, it is possible that N. japonicus have been present at these localities for much longer; on the other hand, its occurrence in the River Dyje floodplain in 2013 was notably new. This introduction was most likely linked to the invasion of L. gibbosus during intense flooding in previous years (Ondračková et al., 2019), since intensive parasitological surveys in the area over previous decades had failed to confirm the parasite’s occurrence (e.g. Kadlec et al., 2003; Dávidová et al., 2008). More recent data show that N. japonicus has become a common parasite in aquaculture ponds and sport-fishing grounds, particularly in the Vltava and Elbe basins. The South-Bohemian region, known for its extensive aquacultural pond systems for common carp Cyprinus carpio Linnaeus, 1758 production, has recorded several cases in recent years, especially in C. carpio (Table 1), indicating the probable importance of aquacultural trade in the recent distribution of the parasite. It is also interesting that the parasites have often been found around the anal opening in C. carpio (personal observation), unlike other fish species where N. japonicus prefers the base of the dorsal and anal fins (Nagasawa & Obe, 2013; Suárez-Morales et al., 2010; Ondračková et al., 2019).
A local-scale distribution survey of N. japonicus in waterbodies of the Czech Republic confirmed that the parasite is now widely distributed over a range of watersheds, with increasing reports since 2013 probably attributable to more focused research on this parasite species. Similar to the situation in the Czech Republic, Japan has placed increased focus on N. japonicus over recent decades, leading to a series of publications on its occurrence in different regions (Nagasawa et al., 2007; Nagasawa & Inoue, 2012; Nagasawa & Uyeno, 2012; Nagasawa & Obe, 2013; Nagasawa & Sato, 2015, 2016). This highlights the role of intensified research in expanding knowledge on the distribution of this copepod species.
Fish host range and preferences
Neoergasilus japonicus exhibits a broad host range, parasitising many different freshwater fish species (Nagasawa & Uyeno, 2012). This study updates the known host list to 132 native and non-native fish species across 15 orders and 27 families. Similar host diversity has been reported for another invasive copepod parasite, L. cyprinacea, which is found on various fish families, though with a preference for cyprinids (Piasecki et al., 2004; Mhaisen & Abdul-Ameer, 2021). Likewise, the updated global host species list for N. japonicus shows that more than 50% of all fish species (81 spp.) reported as a host are Cypriniformes (Fig. 3; Online Resource 1). This preference for cyprinids, and especially the Leuciscidae, was also evident in our detailed studies, with S. erythrophthalmus and R. rutilus being successfully infected under experimental conditions, and S. erythrophthalmus, silver bream Blicca bjoerkna (Linnaeus, 1758) and A. alburnus being the most frequently infected host species in the field study (Online Resource 3). High prevalence and abundance in S. erythrophthalmus has frequently been observed in other habitats and regions, including Lake Sapanca in Turkey (Soylu & Soylu, 2012; Kus & Soylu, 2013), private ponds in south Wales (Great Britain; Elsheikha & Beech, 2017) or in our own previous study in Moravian sandpits (Kvach et al., 2021). While infections in R. rutilus are also frequent, though at lower abundances (e.g. Tuuha et al., 1992; Knopf & Hölker, 2005; Alekseev et al., 2021), infection of B. bjoerkna and A. alburnus has previously been reported from the Tisza river basin in Slovakia and Hungary only (Hanek, 1968; Pónyi & Molnár, 1969).
The literature search indicated that centrarchid species, such as rock bass Ambloplites rupestris (Rafinesque, 1817), L. gibbosus, L. macrochirus, redear sunfish Lepomis microlophus (Günther, 1859), largemouth bass Micropterus salmoides (Lacepède, 1802) and smallmouth bass Micropterus dolmieu Lacepède, 1802, are also highly vulnerable to N. japonicus. Reports of their susceptibility to N. japonicus are known from both the original range of the fish, i.e. from North America (Hayden & Rogers, 1998; Hudson & Bowen, 2002), and from their introduced populations in Bulgaria, the Czech Republic, Hungary, France, Ukraine and Japan (Pónyi & Molnár, 1969; Nagasawa & Sato, 2015; Nagasawa & Obe, 2015; Ondračková et al., 2019, 2021; Alekseev et al., 2021; Kvach et al., 2023). At some localities, parasite infection reaches very high levels, with up to 100% prevalence and an infection intensity of over 100 parasites per fish (Nagasawa & Obe, 2013; Nagasawa & Sato, 2015; Alekseev et al., 2021). Our field and experimental data confirmed centrarchids (L. gibbosus in our case) as highly susceptible hosts. Moreover, in both the short- and long-term experimental setups, L. gibbosus had a higher parasite prevalence than native cyprinids. Why centrarchids should be so susceptible to N. japonicus, however, remains unanswered and will require further investigation.
As natural selection tends to favour hosts exhibiting high tolerance and low resistance, host preference should align with host competence (Manzoli et al., 2021). Neoergasilus japonicus infections demonstrate a minimal immunological response from the host due to the type of attachment (Avenant-Oldewage et al., 2023). Interestingly, our experimental studies revealed that the parasite actively avoided infecting P. parva (Gobionidae), a fish species natural to N. japonicus (Kim & Choi, 2003) and the species from which N. japonicus was originally described (Harada, 1930). Moreover, there is no existing record in the literature from this host’s non-native range. The East-Asian P. parva was accidentally introduced into Europe in the 1960s through the stocking of Chinese carps (Bănărescu, 1964) and rapidly became established due to secondary introductions (Simon et al., 2015). A generally low parasite load, encompassing parasites native to P. parva, such as monogeneans (Ondračková et al., 2023), has been observed across a broad spectrum of its European populations (Gozlan et al., 2010). This could be attributed to an inherent resistance to parasitic infections as a species, or possibility that selected individuals with higher parasite resistance have reached Europe and spread further. In either scenario, the fish’s capacity to avoid parasites, particularly its natural ones, enhances its competitive advantage over local species.
In addition to fish species consistently reported as host of N. japonicus, various orders exhibit one or two species that also serve as susceptible hosts for this parasite (Online Resource 1), though data, where available, indicate that prevalence or intensity of infection is generally lower (e.g. Edélenyi, 1967; Nagasawa & Uyeno, 2012; Kvach et al., 2020, 2022, this study), further confirming the apparent preference towards specific fish orders mentioned above. As an interesting exception, we observed intensive infection of rainbow trout Oncorhynchus mykiss (Walbaum, 1792). Salmonidae, which naturally favour cold and running waters, have not previously been reported as hosts for N. japonicus, possibly because running waters are thought to represent an unsuitable habitat for the parasite (Nagasawa & Inoue, 2012). Bednarska et al. (2009) demonstrated that, under certain conditions, O. mykiss is highly susceptible to another copepod infection caused by L. cyprinacea, a parasite that, like N. japonicus, preferably infects cyprinids. In our case, the trout were reared in a still-water sport-fishing pond in South Bohemia alongside cyprinids, with C. carpio likely serving as the source of infection. Unfortunately, due to massive infection by Argulus foliaceus (Linnaeus, 1758) and co-infection with N. japonicus, all fish died (Ondračková & Hronek, 2023). While several studies have indicated that N. japonicus does not cause severe pathology in the infected host (Avenant-Oldewage et al., 2023), a recent investigation using scanning electron microscopy and histology revealed that the parasite removes the epidermis and brushes it into the buccal cavity, exposing the dermis to opportunistic diseases (Avenant-Oldewage et al., 2023). In conjunction with other infections, fish that have not previously encountered N. japonicus may suffer severely, as observed in the O. mykiss from Southern Bohemia in this study. Furthermore, in Mexico, N. japonicus have infected locally endangered and threatened fish species, with non-negligible infection parameters (Suárez-Morales et al., 2010). These examples underscore the implication that infection of non-typical hosts may have further negative consequences for their health and, potentially, survival.
Factors contributing to parasite infection and dispersal
Host phylogenetic affiliation is emerging as a crucial factor in determining parasite preference, as discussed earlier. However, this and other studies have found host species from a range of fish orders and families infected by N. japonicus (Online Resource 2). Utilising field data on parasite presence and absence across a diverse range of fish species at five sites, our analysis revealed that piscivorous fish were less likely to be infected than benthivorous and planktivorous species. Additionally, pelagic and benthopelagic fish were more prone to infection than demersal species. Similarly, Piasecki et al. (2004) confirmed that planktophagy significantly increased the probability of being parasitised by infectious stages of ergasilids. The risk of infection was also heightened in species inhabiting the water column, where fish hosts may come into direct contact with the parasite more frequently than demersal fish, which reside on or near the bottom of the lake. Our findings align with those of Nagasawa and Inoue (2012), who observed signs of infection in benthopelagic fish but not benthic fish.
Most studies indicate that the parasitic copepod N. japonicus has achieved widespread distribution worldwide; however, this extensive geographical spread cannot be directly attributed to natural dispersion (Hudson & Bowen, 2002; Suárez-Morales & Mercado-Salas, 2013); rather, it appears to have been through the introduction of exotic fish associated with the aquacultural industry. Indeed, translocation with aquacultural species has been proposed as the most likely route of N. japonicus introduction in Cuba (Prieto et al., 1985), the USA (Hudson & Bowen, 2002), Mexico (Suárez-Morales et al., 2010) and the Czech Republic (Ondračková & Hronek, 2023). Ability to parasitise a wide range of host species, particularly those of commercial interest can, therefore, be a key feature accelerating its global spread (Suárez-Morales & Mercado-Salas, 2013). Moreover, in Italy, sport-fishing activities have been hypothesised as a potential source of parasite introduction (Alfonso & Belmonte, 2010), while in Iran, the parasite was introduced via the ornamental fish trade (Mirzaei et al., 2016). In the USA, the spread of N. japonicus around the Great Lakes was likely facilitated by the sale of infected baitfish to anglers fishing in and around the lakes (Marshall et al., 2019).
The spread of N. japonicus also appears to have been facilitated by irresponsible or unintentional introductions of non-indigenous fish species, which may be highly susceptible to the parasite. This appears to have been the case regarding introduction with centrarchids in the Czech Republic. Lepomis gibbosus first appeared in sandpits in the Morava river basin (Table 1) following extensive flooding in the 1990s, and went on to establish stable populations (Konečná et al., 2015). In the 2000s, local anglers transported a couple of specimens to a local oxbow. This unintended introduction also transported N. japonicus to the new location, where it became established and switched from L. gibbosus to other species, such as S. erythrophthalmus, S. cephalus and perch Perca fluviatilis Linnaeus, 1758.
Pónyi & Molnár (1969) identified the small size of the parasite and its ability to release and change fish host as factors supporting the spread of N. japonicus. These authors also noted that only non-ovigerous females could release and change host. However, as mentioned in the introduction, we recorded several ovigerous females releasing themselves from sacrificed fish fin tissue during manipulation and swimming freely in the Petri dish, and similar behaviour has also been observed for a related species, Paraergasilus longidigitus Yin, 1954. This suggests that, when the fish’s body surface is mechanically disturbed, whether by a veterinarian or scientist in the laboratory or by a predatory fish in nature, N. japonicus can detach from the fish and swim freely in the surrounding environment. Although experimental re-infection of ovigerous females in our study demonstrated that they are also capable of re-attaching to a host under experimental conditions, success was very low. It remains to be seen, however, whether ovigerous females have a chance of re-attachment also in the wild.
Despite the various factors supporting spread of N. japonicus mentioned above, there are environmental limitations that may prevent the species becoming as widely distributed as the invasive copepod L. cyprinacea. For example, while L. cyprinacea has been observed in a wide variety of habitats (e.g. Plaul et al., 2010), N. japonicus appears to prefer standing or low-flow waters (Nagasawa & Inoue, 2012), reducing the risk of parasite dispersal via drift. Furthermore, the species has consistently been found in freshwater environments only, suggesting a low tolerance to salinity and an inability to disperse across saline waters (see Hayden & Rogers, 1998), limiting its ability to expand its range and representing a further constraint on the species’ dispersal ability.
Conclusions
Overall, this study revealed a large-scale expansion of the distribution area of N. japonicus over the last decade at both a global and local scale. Despite unusually broad host range of this parasite, our study confirmed certain level of host preference or avoidance towards particular taxonomic or ecological groups of fish. Our findings align with the general suggestions that both the invasive parasites’ distribution and their host spectrum may be broader than currently known. This trend underscores the need for ongoing monitoring and research in all regions globally. We also showed the ability of ovigerous females to re-attach to another host, though very limited, under experimental conditions, potentially increasing parasite invasion success. Finally, field investigation confirmed common occurrence of N. japonicus at sites associated with fishing or aquaculture activities. As aquaculture has been identified as a primary vector for parasite dispersal, further research is needed to comprehensively evaluate the risk of the species’ escape and dispersal mechanisms. This should include an in-depth assessment of the importance of intentional or unintentional species introduction, natural dispersal through interconnected waterbodies and sensitivity to water currents. An increase in interest and recognition of the ecological impacts and potential consequences associated with introduction of non-native parasites into diverse aquatic ecosystems may then help prevent similar scenarios in other related parasite species.
Data availability
The data that support the findings of this study are available in Supplementary material (Online Resource 1–3) and from the corresponding author upon reasonable request.
References
Alekseev, V., C. Cuoc, D. Jamet, J.-L. Jamet & R. Chappaz, 2021. Biological invasion of fish parasite Neoergasilus japonicus (Harada, 1930) (Copepoda: Ergasilidae) in Lake Grand Laoucien, France: a field study on life cycle parameters and reasons for unusual high population density. Life 11: 1100. https://doi.org/10.3390/life11101100.
Alfonso, G. & G. Belmonte, 2010. Neoergasilus japonicus (Harada, 1930): A NEW NON-INDIGENOUS COPEPOD for the Italian fauna. Italian Journal of Zoology 77: 172–178. https://doi.org/10.1080/11250001003591783.
Avenant-Oldewage, A., K. Nagasawa, Q. M. Dos Santos & W. H. Oldewage, 2023. Pathology caused by introduced Neoergasilus japonicus (Copepoda: Ergasilidae) to the skin of indigenous Tilapia sparrmanii in South Africa and scanning electron microscopy study of wound-inflicting structures. Journal of Fish Diseases 47: 1–10. https://doi.org/10.1111/jfd.13867.
Bănărescu, P., 1964. Fauna Republicii Populare Române. 13. Pisces – Osteichthyes (peşti ganoizi şi osoşi), Vol. 13. Academiei Republicii Populare Române, Bucuresti (in Romanian).
Bates, D., M. Maechler, B. Bolker & S. Walker, 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67: 1–48. https://doi.org/10.18637/jss.v067.i01.
Bednarska, M., M. Bednarski, Z. Sołtysiak & R. Polechoński, 2009. Invasion of Lernaea cyprinacea in rainbow trout (Oncorhynchus mykiss). Acta Scientiarum Polonorum, Medicina Veterinaria 8: 27–32.
Bell, A. H., L. R. Katona & N. M. Vellequette, 2023. Development and application of a risk assessment tool for aquatic invasive species in the international Rainy-Lake of the Woods Basin, United States and Canada: U.S. Geological Survey Open-File Report 2022-1070, 26 pp.
Betancur-R, R., E. O. Wiley, G. Arratia, A. Acero, N. Bailly, M. Miya, G. Lecointre & G. Orti, 2017. Phylogenetic classification of bony fishes. BMC Evolutionary Biology 17: 162. https://doi.org/10.1186/s12862-017-0958-3.
Berrouk, H., N. Khelifi, M. Tourafia & C. Boualleg, 2019. Effect of abiotic factors on copepod parasites from Beni-haroun dam (Mila city) north-east of Algeria. Journal of Harmonized Research in Applied Sciences 7: 112–122. https://doi.org/10.30876/JOHR.7.4.2019.112-122.
Bush, A. O., K. D. Lafferty, J. M. Lotz & A. W. Shostak, 1997. Parasitology meets ecology on its own terms: Margolis et al. revisited. Journal of Parasitology 83: 575–583. https://doi.org/10.2307/3284227.
Dávidová, M., M. Ondračková, P. Jurajda & M. Gelnar, 2008. Parasite assemblages of European bitterling (Rhodeus amarus), composition and effects of habitat type and host body size. Parasitology Research 102: 1001–1011. https://doi.org/10.1007/s00436-007-0867-2.
Edélenyi, B., 1967. A Tisza halaiban eloskodo fergek es dinamicus je lentkezesuk. Tudományos Kozlemény 3: 13–42 ((in Hungarian)).
Elsheikha, H. & A. Beech, 2017. Hitching rides on fish: crustaceans revealed in a pond in south Wales. Vet Times. https://www.vettimes.co.uk/app/uploads/wp-post-to-pdf-enhanced-cache/1/hitching-rides-on-fish-crustaceans-revealed-in-a-pond-in-south-wales.pdf.
Ergens, R., V. A. Gussev, N. A. Izumova & K. Molnár, 1975. Parasite Fauna of Fishes of the Tisa River Basin. Rozpravy ČSAV, Academia, Prague, 85, No. 2, 117 pp.
Gao, H.-W., J.-X. Wu, M. Liu, Y. Y. Chen & X. R. Qi, 2010. Diagnosis and cure of Neoergasilus japonicus disease of Carassius auratus. Journal of Economic Animal 14: 46–48.
Gozlan, R. E., D. Andreou, T. Asaeda, K. Beyer, R. Bouhadad, D. Burnard, N. Caiola, P. Cakic, V. Djikanovic, H. R. Esmaeili, I. Falka, D. Golicher, A. Harka, G. Jeney, V. Kováč, J. Musil, A. Nocita, M. Povž, N. Poulet, T. Virbickas, C. Wolter, A. S. Tarkan, E. Tricarico, T. Trichkova, H. Verreycken, A. Witkowski, C. G. Zhang, I. Zweimueller & J. R. Britton, 2010. Pan-continental invasion of Pseudorasbora parva: towards a better understanding of freshwater fish invasions. Fish and Fisheries 11: 315–340. https://doi.org/10.1111/j.1467-2979.2010.00361.x.
Hanek, J., 1968. The finding of Neoergasilus japonicus (Harada, 1930) (Copepoda: Ergasilidae) in Europe. Folia Parasitologica 15: 227–228.
Harada, I., 1930. Studies on fresh-water fauna of Formosa (I). A new copepod species parasitic on Formosan fresh-water fishes. Journal of Tropical Agriculture 2: 71–77.
Hayden, K. J. & W. A. Rogers, 1998. Neoergasilus japonicus (Poecilostomatoida: Ergasilidae), a parasitic copepod new to North America. The Journal of Parasitology 84: 88–93.
Hoberg, E. P., 2010. Invasive processes, mosaics and the structure of helminth parasite faunas. Revue Scientifique et Technique 29: 255–272. https://doi.org/10.20506/rst.29.2.1972.
Hothorn, T., F. Bretz & P. Westfall, 2008. Simultaneous inference in general parametric models. Biometrical Journal 50: 346–363. https://doi.org/10.1002/bimj.200810425.
Hudson, P. L. & C. A. Bowen, 2002. First record of Neoergasilus japonicus (Poecilostomatoida: Ergasilus), a parasitic copepod new to the Laurentian Great Lakes. Journal of Parasitology 88: 657–663. https://doi.org/10.2307/3285339.
Kadlec, D., A. Šimková, J. Jarkovský & M. Gelnar, 2003. Parasite communities of freshwater fish under flood conditions. Parasitology Research 89: 272–283. https://doi.org/10.1007/s00436-002-0740-2.
Kim, I.-H. & S.-K. Choi, 2003. Copepod parasites (Crustacea) of freshwater fishes in Korea. The Korean Journal of Systematic Zoology 19: 57–93.
Kmentová, N., M. Van Steenberge, D. F. E. Thys van den Audenaerde, T. Nhiwatiwa, F. M. Bukinga, T. M. N’sibula, M. M. Pascal, M. Gelnar & M. P. M. Vanhove, 2019. Co-introduction success of monogeneans infecting the fisheries target Limnothrissa miodon differs between two non-native areas: the potential of parasites as a tag for introduction pathway. Biological Invasions 21: 757–773.
Knopf, K. & F. Hölker, 2005. First report of Philometra obturans (Nematoda) and Neoergasilus japonicus (Copepoda) in Germany. Acta Parasitologica 50: 261–262.
Kolar, C. S. & D. M. Lodge, 2001. Progress in invasion biology: predicting invaders. Trends in Ecology and Evolution 16: 199–204. https://doi.org/10.1016/s0169-5347(01)02101-2.
Konečná, M., M. Janáč, K. F. Roche & P. Jurajda, 2015. Variation in life-history traits between a newly established and long-established population of non-native pumpkinseed, Lepomis gibbosus (Actinopterygii: Perciformes: Centrarchidae). Acta Ichthyologica Et Piscatoria 45: 385–392. https://doi.org/10.3750/AIP2015.45.4.06.
Kuchta, R., A. Choudhury & T. Scholz, 2018. Asian fish tapeworm: the most successful invasive parasite in freshwaters. Trends in Parasitology 34: 511–523. https://doi.org/10.1016/j.pt.2018.03.001.
Kumari, P. S., R. Madhavi & R. Ramakrishna, 2009. Neoergasilus japonicus (Harada) (Poecilostomatoida, Ergasilidae), a parasitic copepod new to India. Indian Journal of Fisheries 56(4): 287–291.
Kus, U. S. & E. Soylu, 2013. Metazoan parasites of rudd Scardinius erythrophthalmus in Lake Sapanca, Turkey. Bulletin of the European Association of Fish Pathologists 33: 105–110. https://doi.org/10.35864/evmd.943270.
Kvach, Y., Y. Kutsokon, A. Roman, A. Ceirans, M. Pupins & M. Kirjusina, 2020. Parasite acquisition by the invasive Chinese sleeper (Perccottus glenii Dybowski, 1877) (Gobiiformes: Odontobutidae) in Latvia and Ukraine. Journal of Applied Ichthyology 36: 785–794. https://doi.org/10.1111/jai.14100.
Kvach, Y., M. Y. Tkachenko, M. Seifertová & M. Ondračková, 2021. Insights into the diversity, distribution and phylogeny of three ergasilid copepods (Hexanauplia: Ergasilidae) in lentic water bodies of the Morava river basin, Czech Republic. Limnologica 91: 125922. https://doi.org/10.1016/j.limno.2021.125922.
Kvach, Y., Y. Kutsokon, M. Janáč, I. Dykyy, N. Dzyziuk, I. Dudliv & K. Nazaruk, 2022. Parasites of the Invasive Chinese sleeper Perccottus glenii (Actinopterygii: Odontobutidae) in the region of the first introduction of the Carpathian population. Oceanological and Hydrobiological Studies 51: 1–9.
Kvach, Y., M. Y. Tkachenko, V. Bartáková, Y. Kutsokon, M. Janáč, V. Demchenko & M. Ondračková, 2023. Parasite communities and genetic structure of non-native pumpkinseed, Lepomis gibbosus, in different Black Sea drainages of Ukraine. Knowledge and Management of Aquatic Ecosystems. https://doi.org/10.1051/kmae/2022023.
Lescher-Moutoué, F., 1979. Presence en France du Copepode Ergasilidae Neoergasilus japonicus (Harada), Crustaceana 37. E. J. Brill, Leiden.
Lymbery, A. J., M. Morine, H. G. Kanani, S. J. Beatty & D. L. Morgan, 2014. Co-invaders: the effects of alien parasites on native hosts. International Journal for Parasitology: Parasites and Wildlife 3: 171–177. https://doi.org/10.1016/j.ijppaw.2014.04.002.
MacLeod, C. J., A. M. Paterson, D. M. Tompkins & R. P. Duncan, 2010. Parasites lost – do invaders miss the boat or drown on arrival? Ecology Letters 13: 516–527. https://doi.org/10.1111/j.1461-0248.2010.01446.x.
Manzoli, D. E., M. J. Saravia-Pietropaolo, S. I. Arce, A. Percara, L. R. Antoniazzi & P. M. Beldomenico, 2021. Specialist by preference, generalist by need: Availability of quality hosts drives parasite choice in a natural multihost–parasite system. International Journal for Parasitology 51: 527–534. https://doi.org/10.22541/au.157712391.14898348.
Marshall, C. C., P. L. Hudson, J. R. Jackson, J. K. Connolly, J. M. Watkins & L. G. Rudstam, 2019. First record of the non-indigenous parasitic copepod Neoergasilus japonicus (Harada, 1930) in the Lake Ontario watershed: Oneida Lake, New York. Journal of Great Lakes Research 45: 1348–1353. https://doi.org/10.1016/j.jglr.2019.09.017.
Mendes Marques, T. & G. A. Murrieta Morey, 2018. First record of Neoergasilus japonicus (Harada, 1930) (Copepoda: Cyclopoida) infecting a fish species in South America. Folia Amazónica, Revista del Instituto de Investigaciones de la Amazonía Peruana 27: 111–117. https://doi.org/10.24841/fa.v27i1.460.
Mhaisen, F. T. & K. N. Abdul-Ameer, 2021. Checklist of fish hosts of species of Lernaea Linnaeus, 1758 (Hexanauplia: Cyclopoida: Lernaeidae) in Iraq. Biological and Applied Environmental Research 5: 53–73. https://doi.org/10.51304/baer.2021.5.1.53.
Mirzaei, M., H. Khovand & R. Kheirandish, 2016. The prevalence of non-indigenous parasitic copepod (Neoergasilus japonicus) spreads with fishes of pet trade in Kerman. Iran. Journal of Parasitic Diseases 40: 1283–1288. https://doi.org/10.1007/s12639-015-0669-x.
Moravec, F., 2001. Checklist of Metazoan Parasites of Fishes of the Czech Republic and the Slovak Republic (1873–2000), Academia, Praha:
Mugridge, R. E. R., H. G. Stallybrass & A. Hollman, 1982. Neoergasilus japonicus (Crustacea Ergasilidae). A parasitic copepod new to Britain. Journal of Zoology 197: 551–557. https://doi.org/10.1111/jzo.1982.197.4.551.
Nagasawa, K. & A. Inoue, 2012. Variations in the infection level of Neoergasilus japonicus (Copepoda: Ergasilidae) between freshwater fishes at different sites in the Ashida River system, western Japan. Zoosymposia 8: 107–118.
Nagasawa, K. & D. Uyeno, 2012. Utilization of alien freshwater fishes by the parasitic copepod Neoergasilus japonicus (Ergasilidae) on Okinawa-jima Island, Japan, with a list of the known hosts. Zoosymposia 8: 82–97. https://doi.org/10.11646/zoosymposia.8.1.11.
Nagasawa, K. & M. Obe, 2013. Spatial distribution of Neoergasilus japonicus (Copepoda: Ergasilidae) on the fins of bluegill (Lepomis macrochirus). Journal of Natural History 47: 543–552. https://doi.org/10.1080/00222933.2012.747635.
Nagasawa K. & M. Obe, 2015. The ergasilid copepod Neoergasilus japonicus infecting smallmouth bass Micropterus dolomieu in central Japan. Biosphere Sciences 54: 65–69.
Nagasawa, K. & H. Sato, 2015. Neoergasilus japonicus (Copepoda: Ergasilidae) parasitic on two alien freshwater fishes (Lepomis macrochirus and Micropterus salmoides) in central Japan, with its new record from Gunma Prefecture. Bulletin of the Gunma Museum of Natural History 19: 1–4.
Nagasawa, K. & H. Sato, 2016. Occurrence of two copepods, Lernaea cyprinacea (Lernaeidae) and Neoergasilus japonicus (Ergasilidae), infecting freshwater fishes in Lake Jonuma, Gunma Prefecture, Central Japan. Bulletin of the Gunma Museum of Natural History 20: 161–164.
Nagasawa, K., T. Umino, D. Uyeno & S. Ohtsuka, 2007. A check-list of ergasilid copepods (Crustacea) occurring as fish parasites or plankton in Japan (1895–2007). Bulletin of the Biogeographical Society of Japan 62: 43–62 (In Japanese with English abstract).
Ondračková, M. & J. Hronek, 2023. Asijský chlopek. Nový nepůvodní parazit v našich vodách. Rybářství 11: 60–63 (in Czech).
Ondračková, M., J. Fojtů, M. Seifertová, Y. Kvach & P. Jurajda, 2019. Non-native parasitic copepod Neoergasilus japonicus (Harada, 1930) utilizes non-native fish host Lepomis gibbosus (L.) in the floodplain of the River Dyje (Danube basin). Parasitology Research 118: 57–62. https://doi.org/10.1007/s00436-018-6114-1.
Ondračková, M., V. Bartáková, Y. Kvach, A. Bryjová, T. Trichkova, F. Ribeiro, L. Carassou, A. Martens, G. Masson, T. Zechmeister & P. Jurajda, 2021. Parasite infection reflects host genetic diversity among non-native populations of pumpkinseed sunfish in Europe. Hydrobiologia 848: 2169–2187. https://doi.org/10.1007/s10750-020-04410-y.
Ondračková, M., M. Seifertová, M. Y. Tkachenko, L. Vetešník, H. Liu, V. Demchenko & Y. Kvach, 2023. The parasites of a successful invader: monogeneans of the Asian topmouth gudgeon Pseudorasbora parva, with description of a new species of Gyrodactylus. Parasite 30: 22. https://doi.org/10.1051/parasite/2023024.
Orozco Zamorano, A. 2001. Estudios sobre la estacionalidad de intrapoblaciones de Neoergasilus japonicus (copepoda: Ergasilidae) en peces nativos de El Batán, Qro. Bachelor Thesis, Universidad Auténoma de Querétaro.
Peeler, E. J., B. C. Oidtmann, P. J. Midtlyng, L. Miossec & R. E. Gozlan, 2011. Non-native aquatic animals introductions have driven disease emergence in Europe. Biological Invasions 13: 1291–1303. https://doi.org/10.1007/s10530-010-9890-9.
Piasecki, W., A. E. Goodwin, J. C. Eiras & B. F. Nowak, 2004. Importance of Copepoda in freshwater aquaculture. Zoological Studies 43: 193–205.
Plaul, S. E., N. García Romero & C. G. Barbeito, 2010. Distribution of the exotic parasite, Lernaea cyprinacea (Copepoda, Lernaeidae) in Argentina. Bulletin of the European Association of Fish Pathologists 30: 65.
Pónyi, J. & K. Molnár, 1969. Studies on the parasite fauna of fish in Hungary. V. Parasitic copepods. Parasitologica Hungarica 2: 137–148.
Poulin, R., B. R. Krasnov, D. Mouillot & D. W. Thieltges, 2011. The comparative ecology and biogeography of parasites. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences 366: 2379–2390. https://doi.org/10.1098/rstb.2011.0048.
Prieto, A., E. Fajer & M. Vincoy, 1985. Neoergasilus japonicus (Copepoda:Ergasilidae) en peces en coltivo intensivo en Cuba. Revista De Salud Animal 7: 407–410 ((in Spanish)).
R Core Team 2021. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Sakai, A. K., F. W. Allendorf, J. S. Holt, D. M. Lodge, J. Molofsky, K. A. With, S. Baughman, R. J. Cabin, J. E. Cohen, N. C. Ellstrand, D. E. McCauley, P. O’Neil, I. M. Parker, J. N. Thompson & S. G. Weller, 2001. The population biology of invasive species. Annual Review of Ecology and Systematics 32: 305–332. https://doi.org/10.1146/annurev.ecolsys.32.081501.114037.
Šimková, A., E. Řehulková, J. R. Rasoloariniaina, M. W. P. Jorissen, T. Scholz, A. Faltýnková, Š Mašová & M. P. M. Vanhove, 2019. Transmission of parasites from introduced tilapias: a new threat to endemic Malagasy ichtyofauna. Biological Invasions 21: 803–819. https://doi.org/10.1007/s10530-018-1859-0.
Simon, A., R. E. Gozlan, J. R. Britton, C. van Oosterhout & B. Hänfling, 2015. Human induced step**-stone colonisation of an admixed founder population: the spread of topmouth gudgeon (Pseudorasbora parva) in Europe. Aquatic Sciences 77: 17–25. https://doi.org/10.1007/s00027-014-0374-3.
Smirnova, T. S., 1971. Parasitic Crustacea from the fishes of the river Amur’s basin. Parasitology Paper 25: 177–195.
Soylu, E. & M. P. Soylu, 2012. First record of the non-indigenous parasitic copepod Neoergasilus japonicus (Harada, 1930) in Turkey. Turkish Journal of Zoology 36: 662–667. https://doi.org/10.3906/zoo-1101-92.
Suárez-Morales, E. & N. Mercado-Salas, 2013. The non-indigenous parasitic copepod Neoergasilus japonicus (Harada) (Cyclopoida) from central Mexico: the earliest invasion in continental America. BioInvasions Records 2: 201–206. https://doi.org/10.3391/bir.2013.2.3.05.
Suárez-Morales, E., A. Paredes-Trujillo & D. González-Solís, 2010. The introduced Asian parasitic copepod Neoergasilus japonicus (Harada) (Cyclopoida: Ergasilidae) from endangered Cichlid teleosts in Mexico. Zoological Science 27: 851–855. https://doi.org/10.2108/zsj.27.851.
Taraschewski, H., 2006. Hosts and parasites as aliens. Journal of Helminthology 80: 99–128. https://doi.org/10.1079/JOH2006364.
Torchin, M. E., K. D. Lafferty, A. P. Dobson, V. J. McKenzie & A. M. Kuris, 2003. Introduced species and their missing parasites. Nature 421: 628–629. https://doi.org/10.1038/nature01346.
Truong, T. N. & S. A. Bullard, 2021. Susceptibility of channel catfish (Ictalurus punctatus), blue catfish (Ictalurus furcatus), and their commercially cultured hybrid to metazoan parasite infection in earthen pond aquaculture. Comparative Parasitology 88: 93–112. https://doi.org/10.1654/1525-2647-88.1.93.
Truter, M., K. A. Hadfield & N. J. Smit, 2023. Parasite diversity and community structure of translocated Clarias gariepinus (Burchell) in South Africa: testing co-introduction, parasite spillback and enemy release hypotheses. International Journal for Parasitology: Parasites and Wildlife 20: 170–179. https://doi.org/10.1016/j.ijppaw.2023.02.004.
Tuuha, H., E. T. Valtonen & J. Taskinen, 1992. Ergasilid copepods as parasites of perch Perca fluviatilis and roach Rutilus rutilus in Central Finland seasonality, maturity and environmental influence. Journal of Zoology 228: 405–422. https://doi.org/10.1111/j.1469-7998.1992.tb04444.x.
Urawa, S., K. Muroga & S. Kasahara, 1980. Studies on Neoergasilus japonicus (Copepoda: Ergasilidae), a parasite of freshwater fishes. 2. Development in copepodid stage. Journal of the Faculty of Applied Biological Science, Hiroshima University 19: 21–38.
Urawa, S., K. Muroga, S. Kashara, 1991. Growth and fecundity of the parasitic copepod Neoergasilus japonicus (Ergasilidae). Bulletin of Plankton Society of Japan in Proceedings of the International Conference on Copepoda 4: 619–625.
Velázquez-Ornelas K., E. Juárez-Carrillo & M. Ayón-Parente, 2021. Zooplancton (Cladocera Y Copepoda) De La Laguna De Cajititlán. e-CUCBA 16: 12–20.
Waicheim, M. A., M. Arbetman, C. Rauque & G. Viozzi, 2019. The invasive parasitic copepod Lernaea cyprinacea: updated host-list and distribution, molecular identification and infection rates in Patagonia. Aquatic Invasions 14: 350–264. https://doi.org/10.3391/ai.2019.14.2.12.
Wang, K.-N., 1964. Parasitic crustaceans of fresh water fishes in Kiangsu and Shanghai. Acta Zoologica Sinica 16: 465–473.
Welicky, R. L., J. De Swardt, R. Gerber, E. C. Netherlands & N. J. Smit, 2017. Drought-associated absence of alien invasive anchorworm, Lernaea cyprinacea (Copepoda: Lernaeidae), is related to changes in fish health. International Journal for Parasitology: Parasites Wildlife 6: 430–438. https://doi.org/10.1016/j.ijppaw.2017.01.004.
Yin, W. Y., 1956. Studies on the Ergasilidae (Parasitic Copepoda) from the fresh-water fishes of China. Acta Hydrobiologia Sinica 2: 219–270.
Zeileis, A. & T. Hothorn, 2002. Diagnostic checking in regression relationships. R News 2: 7–10.
Acknowledgements
We thank our colleagues from the Czech Academy of Sciences (Institute of Vertebrate Biology) for their support during fish sampling and parasite processing and the representatives of the Czech and Moravian Angling Associations for the opportunity to sample fish in their waters. We also thank Kevin Roche for proofreading the English text.
Funding
Open access publishing supported by the National Technical Library in Prague. This study was financially supported by the Czech Science Foundation, project No. 20-29111S (experimental studies) and 23-07185S (field studies).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Ethical approval
This research was undertaken in line with the ethical requirements of the Czech Republic and has been approved by the appropriate ethics committee (Permit No. MO-2017-02). The maintenance and care of fish, as well as the method of experimental infection and fish killing, complied with the legal requirements of the Czech Republic (§ 7 law No. 114/1992 on the Protection of Nature and Landscape and § 6, 7, 9 and 10 regulation No. 419/2012 on the Care, Breeding and Use of Experimental Animals). MO is certified according to Czech legal requirements (§15, Law No. 246/1992 on Animal Welfare) to work with experimental animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Sidinei M. Thomaz, Cécile Fauvelot, Lee B. Kats, Jonne Kotta & Fernando M. Pelicice / Aquatic Invasive Species IV
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ondračková, M., Tkachenko, M.Y., Vetešník, L. et al. Distribution and host range of a highly invasive parasitic copepod. Hydrobiologia (2024). https://doi.org/10.1007/s10750-024-05577-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10750-024-05577-4