Abstract
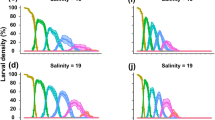
We investigated the effects of eight different levels of salinity (0–35) on the larval development of Upogebia vasquezi, while the abundance of the larvae within the Marapanim estuary on the Amazon Coast was verified through the monthly collection of specimens between August 2006 and July 2007. This species reproduces year-round on the Amazon Coast, which is subjected to strong seasonal fluctuations in salinity due to the local precipitation regime. Upogebia vasquezi larvae developed optimally in salinity close to that of seawater (20–35), while low salinities (0, 5, and 10) did not support the survival of the larvae. Only zoeal stages I, II, and III were captured in the field and were more abundant at the higher end of the salinity gradient, in the areas closest to the adjacent coastal waters. Data from both the laboratory and the field data emphasized the low survival potential of the larvae in low salinities, and increased survival and improved development in more saline water. These results support the hypothesis that U. vasquezi undergo development on the shelf, and also suggest the possibility of an ontogenetic migration toward to adjacent coastal areas during early larval stages, as observed in other decapod species around the world.





Similar content being viewed by others
References
Anger, K., 1991. Effects of temperature and salinity on the larval development of the Chinese mitten crab Eriocheir sinensis (Decapoda: Grapsidae). Marine Ecology Progress Series 72: 103–110.
Anger, K., 1996. Salinity tolerance of the larvae and first juveniles of a semiterrestrial grapsid crab, Armases miersii (Rathbun). Journal of Experimental Marine Biology and Ecology 202: 205–223.
Anger, K., 2001. The biology of decapod crustacean larvae, Vol. 14., Crustacean Issues A. A. Balkema Publishers, Lisse.
Anger, K., 2003. Salinity as a key parameter in the larval biology of decapods crustaceans. Invertebrate Reproduction and Development 43(1): 29–45.
Anger, K., E. Spivak, C. Bas, D. Ismael & T. Luppi, 1994. Hatching rhythms and dispersion of decapod crustacean larvae in a brackish coastal lagoon in Argentina. Helgolander Meeresuntersuchungen 48: 445–466.
Anger, K., E. Spivak, T. Luppi, C. Bas & D. Ismael, 2008. Larval salinity tolerance of the South American salt-marsh crab, Neohelice (Chasmagnathus) granulata: physiological constraints to estuarine retention, export and reimmigration. Helgoland Marine Research 62: 93–102.
Ayres, M., M. Ayres Jr, D. L. Ayres & A. S. Santos, 2007. BioEstat 5.0 Aplicações estatísticas nas áreas das ciências biológicas e médicas. Instituto do desenvolvimento sustentável Mamirauá – IDSM/MCT/CNPq, Pará.
Berrêdo, J. F., M. L. Costa & M. P. S. Progene, 2008. Efeitos das variações sazonais do clima tropical úmido sobre as águas e sedimentos de manguezais do estuário do rio Marapanim, costa nordeste do Estado do Pará. Acta Amazonica 38(3): 473–482.
Boschi, E. E., 1981. Larvas de Crustacea Decapoda. In Boltovskoy, D. (ed.), Atlas del zooplancton del Atlántico sudoccidental y métodos de trabajo con el zooplancton marino. Inedep, Mar del Plata.
Charmantier, G., 1998. Ontogeny of osmoregulation in crustaceans: a review. Invertebrate Reproduction and Development 33: 177–190.
Charmantier, G., L. Giménez, M. Charmantier-Daures & K. Anger, 2002. Ontogeny of osmoregulation, physiological plasticity and larval export strategy in the grapsid crab Chasmagnathus granulata (Crustacea, Decapoda). Marine Ecology Progress Series 229: 185–194.
Christy, J. H. & S. G. Morgan, 1998. Estuarine immigration by crab postlarvae: mechanisms, reliability and adaptive significance. Marine Ecology Progress Series 174: 51–65.
Costlow, J. D., C. G. Bookhout & R. Monroe, 1960. The effect of salinity and temperature on larval development of Sesarma cinereum (Bosc) reared in the laboratory. Biological Bulletin 118(2): 183–202.
Diele, K. & D. J. B. Simith, 2006. Salinity tolerance of northern Brazilian mangrove crab larvae, Ucides cordatus (Ocypodidae): necessity for larval export? Estuarine, Coastal and Shelf Science 68: 600–608.
Dworschak, P. C., 1988. The biology of Upogebia pusilla (Petagna) (Decapoda, Thalassinidea) III. Growth and production. Marine Ecology 9(1): 51–77.
Dworschak, P. C., 2005. Global diversity in the Thalassinidea (Decapoda): an update (1998–2004). Nauplius 13(1): 57–63.
Dworschak, P. C., D. L. Felder & C. C. Tudge, 2012. Infraorders Axiidea De Saint Laurent, 1979 and Gebiidea De Saint Laurent, 1979 (formerly known collectively as Thalassinidea). In Schran, F. R. & J. C. Vaupel Klein (eds), The Crustacea - Treatise on Zoology: anatomy, taxonomy, biology. Koninklijke Brill NV, Boston: 109–219.
Esser, L. J. & N. Cumberlidge, 2011. Evidence that salt water may not be a barrier to the dispersal of Asian freshwater crabs (Decapoda: Brachyura: Gecarcinucidae and Potamidae). Raffles Bulletin of Zoology 59(2): 259–268.
Faleiro, F., J. Paula & L. Narciso, 2012. Hot and salty: the temperature and salinity preferences of a temperate estuarine shrimp larva, Upogebia pusilla (Decapoda: Thalassinidea). Hydrobiologia 691: 89–95.
Fowler, A. E., N. V. Gerner & M. A. Sewell, 2011. Temperature and salinity tolerances of Stage 1 zoeae predict possible range expansion of an introduced portunid crab, Charybdis japonica, in New Zealand. Biological Invasions 13: 691–699.
Johnson, G. E. & J. J. Gonor, 1982. The tidal exchange of Callianassa californiensis (Crustacea, Decapoda) larvae between the Ocean and the Salmon River estuary, Oregon. Estuarine, Coastal and Shelf Science 14: 501–516.
Melo Jr, M., R. Schwamborn, S. Neumann-Leitão & M. Paranaguá, 2012. Abundance and instantaneous transport of Petrolisthes armatus (Gibbes, 1850) planktonic larvae in the Catuama inlet, Northeast Brazil. Anais da Academia Brasileira de Ciências 84(1): 95–102.
Moraes, B. C., J. M. N. Costa, A. C. L. Costa & M. H. Costa, 2005. Variação espacial e temporal da precipitação no estado do Pará. Acta Amazonica 35(2): 207–214.
Nóbrega, P. S. V., B. Bentes & J. M. Martinelli-Lemos, 2013. Composition of shrimp populations (Crustacea: Decapoda) in non-vegetated areas of two river islands in a Brazilian Amazon estuary. Zoologia 30(6): 652–660.
O’Connor, N. J. & C. E. Epifanio, 1985. The effect of salinity on the dispersal and recruitment of fiddler Crab Larvae. Journal of Crustacean Biology 5(1): 137–145.
Oliveira, D. B., D. C. Silva & J. M. Martinelli-Lemos, 2012. Density of larval and adult forms of the burrowing crustaceans Lepidophthalmus siriboia (Callianassidae) and Upogebia vasquezi (Upogebiidae) in an Amazon estuary, northern Brazil. Journal of the Marine Biological Association of the United Kingdom 92(2): 295–303.
Oliveira, D. B., D. C. Silva & J. M. Martinelli-Lemos, 2013. Larval and adult density of the porcellanid crab Petrolisthes armatus (Anomura: Porcellanidae) in an Amazon estuary, northern Brazil. Zoologia 30(6): 592–600.
Oliveira, D. B., J. M. Martinelli-Lemos & F. A. Abrunhosa, 2014. The complete larval development of the mud shrimp Upogebia vasquezi (Gebiidea: Upogebiidae) reared in the laboratory. Zootaxa 3826(3): 517–543.
Paula, J., R. N. Mendes, S. Paci, P. McLaughlin, F. Gherardi & W. Emmerson, 2001. Combined effects of temperature and salinity on the larval development of the estuarine mud prawn Upogebia africana (Crustacea, Thalassinidea). Hydrobiologia 449: 141–148.
Pires, R. F. T., M. Pan, M. P. Santos, A. Peliz, D. Boutov & A. Santos, 2013. Modelling the variation in larval dispersal of estuarine and coastal ghost shrimp: Upogebia congeners in the Gulf of Cadiz. Marine Ecology Progress Series 492: 153–168.
Sakai, K. & M. Turkay, 2014. A review of the collections of the infraorders Thalassinidea Latreille, 1831 and Callianassidea Dana, 1852 (Decapoda, Pleocyemata) lodged in three German museums, with revised keys to the genera and species. Crustaceana 87(2): 129–211.
Santos, A., A. M. P. Santos, D. V. P. Conwa, C. Bartilotti, P. Lourenço & H. Queiroga, 2008. Diel vertical migration of decapod larvae in the Portuguese coastal upwelling ecosystem: implications for offshore transport. Marine Ecology Progress Series 359: 171–183.
Silva, D. C. & J. M. Martinelli-Lemos, 2012. Species composition and abundance of the benthic community of Axiidea and Gebiidea (Crustacea: Decapoda) in the Marapanim Bay, Amazon estuary, northern Brazil. Zoologia 29(2): 144–158.
Simith, D. J. B. & K. Diele, 2008. O efeito da salinidade no desenvolvimento larval do caranguejo-uçá, Ucides cordatus (Linnaeus, 1763) (Decapoda: Ocypodidae) no Norte do Brasil. Acta Amazonica 38(2): 345–350.
Simith, D. J. B., A. S. Souza, C. R. Maciel, F. A. Abrunhosa & K. Diele, 2012. Influence of salinity on the larval development of the fiddler crab Uca vocator (Ocypodidae) as an indicator of ontogenetic migration towards offshore waters. Helgoland Marine Research 66: 77–85.
Simith, D. J. B., M. A. B. Pires, F. A. Abrunhosa, C. R. Maciel & K. Diele, 2014. Is larval dispersal a necessity for decapod crabs from the Amazon mangroves? Response of Uca rapax zoeae to different salinities and comparison with sympatric species. Journal of Experimental Marine Biology and Ecology 457: 22–30.
Souza Filho, P. W. M., 2005. Costa de manguezais de macromaré da Amazônia: cenários morfológicos, mapeamento e quantificação de áreas usando dados de sensores remotos. Revista Brasileira de Geofísica 23(4): 427–435.
Statsoft Inc., 2004. STATISTICA data analysis software system, version 7, www.statsoft.com.
Thessalou-Legaki, M., 1990. Advanced larval development of Callianassa tyrrhena (Decapoda: Thalassinidea) and the effect of environmental factors. Journal of Crustacean Biology 10(4): 659–666.
Thompson, L. C. & A. W. Pritchard, 1969. Osmoregulatory capacities of Callianassa and Upogebia (Crustacea: Thalassinidea). Biological Bulletin 136(1): 114–129.
Torres, G., L. Giménez & K. Anger, 2011. Growth, tolerance to low salinity, and osmoregulation in decapod crustacean larvae. Aquatic Biology 12: 249–260.
Vuichard, G. S., N. Farías & T. Luppi, 2013. Hatching and larval export of the intertidal crab Neohelice granulata in Mar Chiquita coastal lagoon, Argentina. Iheringia Série Zoologia 103(2): 124–133.
Wooldridge, T. H. & H. Loubser, 1996. Larval release rhythms and tidal exchange in the estuarine mudprawn, Upogebia africana. Hydrobiologia 337: 113–121.
Yannicelli, B., L. R. Castro, A. Valle-Levinson, L. Atkinson & D. Figueroa, 2006. Vertical distribution of decapod larvae in the entrance of an equatorward facing bay of central Chile: implications for transport. Journal of Plankton Research 28(1): 19–37.
Acknowledgments
We thank our colleagues Danielle Arruda, Darlan Simith, Luiz Paulo Melo, Orlando Galli, Rubem Pessoa, and Wellington Trindade for the collection of seawater samples in the field, and Rory Oliveira for hel** to collect ovigerous females. We also appreciate the valuable suggestions of Dr. Cristiana Maciel, Dr. Eduardo Martinelli, Dr. Marcelo Petracco, Dr. Ralf Schwamborn, and Dr. Sandra Shumway, which greatly improved the paper. This study is part of the Ph.D thesis of the first author (DBO) and was funded by the Brazilian National Research Council (CNPq) through the CT-AMAZÔNIA project (Grant 553106/2005-8 to JMML), the Brazilian Higher Education Training Program (CAPES), and the Brazilian Carcinology Society (Grant SBC 01/2012). We also thank PROPESP/FADESP (PAPQ Program) for sponsoring the translation of the original manuscript by Stephen Ferrari. All experiments conducted in this study complied with current Brazilian federal legislation (environmental authorization MMA/ICMBIO/SISBIO Number 29434-5) and Pará state laws.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Vasilis Valavanis
Rights and permissions
About this article
Cite this article
de Oliveira, D.B., Martinelli-Lemos, J.M., de Souza, A.S. et al. Does retention or exportation occur in the larvae of the mud shrimp Upogebia vasquezi (Decapoda, Gebiidea)? Implications for the reproductive strategy of the species on the Amazon coast. Hydrobiologia 773, 241–252 (2016). https://doi.org/10.1007/s10750-016-2708-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2708-8