Abstract
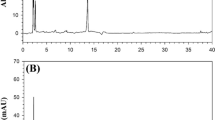
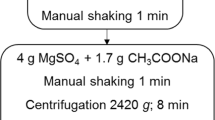
This study presents the use of Quick Easy Cheap Efficient Rugged and Safe (QuEChERS) as an effective sample cleaning procedure and switchable solvent liquid phase microextraction (SS-LPME) as a preconcentration tool for the determination of fenazaquin by gas chromatography mass spectrometry (GC-MS) at ultratrace levels. After a thorough optimization process, 0.50 mL of switchable solvent, 1.5 mL of 1.0 M sodium hydroxide, and 15 s of vortexing were determined as optimum conditions of the SS-LPME method. The limit of detection (LOD) and limit of quantitation (LOQ) determined using the optimum method (SS-LPME/GC-MS) were 0.05 and 0.18 ng/mL, respectively. Compared with direct GC-MS determination of fenazaquin, the optimum method yielded about 800-fold enhancement in detection power of GC-MS. The method was applied to lake, irrigation canal, well, and wastewater samples. In order to test the method’s applicability on fresh tomato samples, a QuEChERS method was used before applying the SS-LPME method. Matrix-matched calibration standards were used to enhance the accuracy of fenazaquin quantification in spiked tomato samples to obtain recovery results close to 100%.




Similar content being viewed by others
References
Abdollahi, M., Ranjbar, A., Shadnia, S., Nikfar, S., & Rezaiee, A. (2004). Pesticides and oxidative stress: A review. Medical Science Monitor, 10(6), RA141–RA147.
Alinejad, M., Kheradmand, K., & Fathipour, Y. (2014). Sublethal effects of fenazaquin on life table parameters of the predatory mite Amblyseius swirskii (Acari: Phytoseiidae). Experimental and Applied Acarology, 64(3), 361–373.
Anastassiades, M., Lehotay, S. J., Štajnbaher, D., & Schenck, F. J. (2003). Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. Journal of AOAC International, 86(2), 412–431.
Anugwom, I., Mäki-Arvela, P., Virtanen, P., Willför, S., Sjöholm, R., & Mikkola, J.-P. (2012). Selective extraction of hemicelluloses from spruce using switchable ionic liquids. Carbohydrate Polymers, 87(3), 2005–2011.
Bonner, M. R., & Alavanja, M. C. (2017). Pesticides, human health, and food security. Food and Energy Security, 6(3), 89–93.
Chen, H., Yin, P., Wang, Q., Jiang, Y., & Liu, X. (2014). A modified QuEChERS sample preparation method for the analysis of 70 pesticide residues in tea using gas chromatography-tandem mass spectrometry. Food Analytical Methods, 7(8), 1577–1587.
Erarpat, S., Özzeybek, G., Chormey, D. S., & Bakırdere, S. (2017). Determination of lead at trace levels in mussel and sea water samples using vortex assisted dispersive liquid-liquid microextraction-slotted quartz tube-flame atomic absorption spectrometry. Chemosphere, 189, 180–185. https://doi.org/10.1016/j.chemosphere.2017.09.072.
Ersoy, N., Tekinarslan, O., Ozgür, E. A., & Göktas, U. (2018). Determination of pesticide residues in apricot (Prunus armeniaca L.) grown at good agricultural practices (GAPs) by LC-MS/MS and GC-MS. Erwerbs-Obstbau, 60(4), 349–358.
Farajzadeh, M. A., Hojghan, A. S., & Mogaddam, M. R. A. (2018). Development of a new temperature-controlled liquid phase microextraction using deep eutectic solvent for extraction and preconcentration of diazinon, metalaxyl, bromopropylate, oxadiazon, and fenazaquin pesticides from fruit juice and vegetable samples followed by gas chromatography-flame ionization detection. Journal of Food Composition and Analysis, 66, 90–97.
Farajzadeh, M. A., Safi, R., & Yadeghari, A. (2019). Combination of QuEChERS extraction with magnetic solid phase extraction followed by dispersive liquid–liquid microextraction as an efficient procedure for the extraction of pesticides from vegetable, fruit, and nectar samples having high content of solids. Microchemical Journal.
Gilden, R. C., Huffling, K., & Sattler, B. (2010). Pesticides and health risks. Journal of Obstetric, Gynecologic & Neonatal Nursing, 39(1), 103–110.
Jessop, P. G., Heldebrant, D. J., Li, X., Eckert, C. A., & Liotta, C. L. (2005). Reversible nonpolar-to-polar solvent. Nature, 436(7054), 1102–1102. https://doi.org/10.1038/4361102a.
Kim, K.-H., Kabir, E., & Jahan, S. A. (2017). Exposure to pesticides and the associated human health effects. Science of the Total Environment, 575, 525–535.
Kumar, V., Tewary, D. K., Ravindranath, S. D., & Shanker, A. (2004). Investigation in tea on fate of fenazaquin residue and its transfer in brew. Food and Chemical Toxicology, 42(3), 423–428.
Lehotay, S. J. (2007). Determination of pesticide residues in foods by acetonitrile extraction and partitioning with magnesium sulfate: Collaborative study. Journal of AOAC International, 90(2), 485–520.
Li, N., Lei, L., Nian, L., Zhang, R., Wu, S., Ren, R., Wang, Y., Zhang, H., & Yu, A. (2013). A modified QuEChERS method for the determination of some herbicides in yogurt and milk by high performance liquid chromatography. Talanta, 105, 219–228.
Łozowicka, B., Rutkowska, E., & Jankowska, M. (2017). Influence of QuEChERS modifications on recovery and matrix effect during the multi-residue pesticide analysis in soil by GC/MS/MS and GC/ECD/NPD. Environmental Science and Pollution Research, 24(8), 7124–7138.
Nicolopoulou-Stamati, P., Maipas, S., Kotampasi, C., Stamatis, P., & Hens, L. (2016). Chemical pesticides and human health: The urgent need for a new concept in agriculture. Frontiers in Public Health, 4, 148–148. https://doi.org/10.3389/fpubh.2016.00148.
Rameshgar, J., Hasheminasab, K. S., Adlnasab, L., & Ahmar, H. (2017). Switchable-hydrophilicity solvent-based microextraction combined with gas chromatography for the determination of nitroaromatic compounds in water samples. Journal of Separation Science, 40(15), 3114–3119.
Rizzetti, T. M., Kemmerich, M., Martins, M. L., Prestes, O. D., Adaime, M. B., & Zanella, R. (2016). Optimization of a QuEChERS based method by means of central composite design for pesticide multiresidue determination in orange juice by UHPLC–MS/MS. Food Chemistry, 196, 25–33.
Sangeetha, S., & Ramaraju, K. (2013). Relative toxicity of fenazaquin against two-spotted spider mite on okra. International Journal of Vegetable Science, 19(3), 282–293.
Silva, M. P., Tubino, M., Elsholz, T. C. R., Elsholz, O., Khan, S., & Vila, M. M. D. C. (2015). Flow injection analysis system for screening organophosphorus pesticides by their inhibitory effect on the enzyme acethylcholinesterase. Journal of the Brazilian Chemical Society, 26, 484–489.
Souza, A. d., Medeiros, A. D. R., Souza, A. C. D., Wink, M., Siqueira, I. R., Ferreira, M. B. C., et al. (2011). Evaluation of the impact of exposure to pesticides on the health of the rural population: Vale do Taquari, State of Rio Grande do Sul (Brazil). Ciência & Saúde Coletiva, 16(8), 3519–3528.
Soylak, M., Khan, M., & Yilmaz, E. (2016). Switchable solvent based liquid phase microextraction of uranium in environmental samples: A green approach. Analytical Methods, 8(5), 979–986. https://doi.org/10.1039/C5AY02631H. https://doi.org/10.1039/C5AY02631H.
Szarka, A., Turková, D., & Hrouzková, S. (2018). Dispersive liquid-liquid microextraction followed by gas chromatography–mass spectrometry for the determination of pesticide residues in nutraceutical drops. Journal of Chromatography A, 1570, 126–134.
Tankiewicz, M., & Biziuk, M. (2018). Fast, sensitive and reliable multi-residue method for routine determination of 34 pesticides from various chemical groups in water samples by using dispersive liquid–liquid microextraction coupled with gas chromatography–mass spectrometry. Analytical and Bioanalytical Chemistry, 410(5), 1533–1550.
Vanderveen, J. R., Durelle, J., & Jessop, P. G. (2014). Design and evaluation of switchable-hydrophilicity solvents. Green Chemistry, 16(3), 1187–1197.
Walorczyk, S., Drożdżyński, D., & Kierzek, R. (2015). Two-step dispersive-solid phase extraction strategy for pesticide multiresidue analysis in a chlorophyll-containing matrix by gas chromatography–tandem mass spectrometry. Journal of Chromatography A, 1412, 22–32.
Wu, M., & Hu, J. (2014). Residue analysis of fosthiazate in cucumber and soil by QuEChERS and GC-MS. Chemical Papers, 68(10), 1368–1374.
Yilmaz, E., & Soylak, M. (2015). Switchable solvent-based liquid phase microextraction of copper (II): Optimization and application to environmental samples. Journal of Analytical Atomic Spectrometry, 30(7), 1629–1635.
Yilmaz, E., & Soylak, M. (2018). A novel and simple deep eutectic solvent based liquid phase microextraction method for rhodamine B in cosmetic products and water samples prior to its spectrophotometric determination. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 202, 81–86.
Zhao, P., Wang, L., Zhou, L., Zhang, F., Kang, S., & Pan, C. (2012). Multi-walled carbon nanotubes as alternative reversed-dispersive solid phase extraction materials in pesticide multi-residue analysis with QuEChERS method. Journal of Chromatography A, 1225, 17–25.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Turan, N.B., Maltepe, E., Chormey, D.S. et al. Determination of fenazaquin in water and tomato matrices by GC-MS after a combined QuEChERS and switchable solvent liquid phase microextraction. Environ Monit Assess 192, 72 (2020). https://doi.org/10.1007/s10661-019-8061-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-8061-4