Abstract
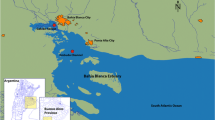
Polycyclic aromatic hydrocarbons (PAHs) are released to the environment from oil sands operations and from natural sources in Alberta, Canada. Concentrations of 16 USEPA priority PAHs were measured in tissues of fishes collected from three locations on the Athabasca River in Alberta and two downstream locations on the Slave River in the Northwest Territories, Canada. A total of 425 individual fish were collected including 89 goldeye (Hiodon alosoides), 93 whitefish (Coregonus clupeaformis), 104 northern pike/jackfish (Esox lucius), 96 walleye (Sander vitreus) and 43 burbot/loche mariah/mariah (Lota lota). Fish were sampled during the summer and fall of 2011 and spring of 2012. Dorsal muscle of fishes from upstream reaches of the Athabasca River, close to oil sands extraction and upgrading activities, contained greater concentrations of individual PAHs than concentrations in muscle of fishes from further downstream in the Slave River. Concentrations of the sum of USEPA indicator PAHs (∑PAHs) in fishes collected in the vicinity of Fort McKay, closest to oil sands activities, varied among seasons with average concentrations ranging from 11 (burbot, summer) to 1.2 × 102 ng/g, wm (burbot, spring) with a mean of 48 ng/g, wm. Concentrations of ∑PAHs in fishes collected in the vicinity of Fort Resolution, the location most distant from oil sands activities, also varied among species and seasons, with average concentrations ranging from 4.3 (whitefish, summer) to 33 ng/g, wm (goldeye, summer) with a mean of 13 ng/g, wm. Significant differences in concentrations of ∑PAHs in muscle were observed within goldeye, jackfish, walleye and whitefish among sites. Health risks posed by PAHs to humans were assessed probabilistically using a B[a]P equivalents approach (B[a]Peq). The average lifetime risk of additional cancers for humans who consumed fish was deemed to be within an ‘acceptable’ range of risk (i.e., less than 10−6).







Similar content being viewed by others
References
Agency for Toxic Substances and Disease Registry (ATSDR). (1996). Minimal risk levels (MRLs) for hazardous substance. Washington, DC: ATSDR.
Ahokas, J. T., & Pelkonen, O. (1984). Metabolic activation of polycyclic aromatic hydrocarbons by fish liver cytochrome P-450. Marine Environmental Research, 14(1–4), 59–69.
Al-Yakoob, S. N., Saeed, T., & Al-Hashash, H. (1994). Polycyclic aromatic hydrocarbons in fish: Exposure assessment for Kuwaiti consumers after the gulf oil spill of 1991. Environment International, 20(2), 221–227.
Berry, E. M. (1997). Dietary fatty acids in the management of diabetes mellitus. American Journal of Clinical Nutrition, 66, 991–997.
Binelli, A., & Provini, A. (2004). Risk for human health of some POPs due to fish from Lake Iseo. Ecotoxicology and Environmental Safety, 58(1), 139–145.
Borga, K. (2011). Trophic magnification factors: Considerations of ecology, ecosystems, and study design. Integrated Environmental Assessment and Management, 8(1), 64–84.
Braune, B. (1999). Spatial and temporal trends of contaminants in Canadian Arctic freshwater and terrestrial ecosystems: a review. Science of the Total Environment, 230(1–3), 145–207.
Chen, Y. (2009). Cancer Incidence in Fort Chipewyan, Alberta 1995–2006. Alberta Cancer Board, Division of Population Health and Information Surveillance, Alberta Health Services. http://www.ualberta.ca/~avnish/rls-2009-02-06-fort-chipewyan-study.pdf. Accessed June 13 2014.
Cheung, K. C., Leung, H. M., Kong, K. Y., & Wong, M. H. (2007). Residual levels of DDTs and PAHs in freshwater and marine fish from Hong Kong markets and their health risk assessment. Chemosphere, 66(3), 460–468.
Conly, M. F., Crosley, R. W., & Headley, J. V. (2002). Characterizing sediment sources and natural hydrocarbon inputs in the lower Athabasca River, Canada. Journal of Environmental Engineering and Science, 1(3), 187–199.
Deutsch-Wenzel, R. P. (1983). Experimental studies in rat lungs on the carcinogenicity and dose–response relationships of eight frequently occurring environmental polycyclic aromatic hydrocarbons. J. National Cancer Institute, 71, 539–544.
Dillon P, Dixon, G.D., Driscoll, C., Giesy, J.P., Hurlbert, S., Nriagu, J. (2011). Evaluation of four reports on contamination of the Athabasca River system by oil sands operations. Prepared by Water Monitoring Data Review Committee. Prepared for Government of Alberta, Canada.
Evans, M. S. (2002). PAH sediment studies in Lake Athabasca and the Athabasca River ecosystem related to the Fort McMurray oil sands operations: sources and trends. In C. A. BrebbiaWIT Press (Ed.), Oil and hydrocarbon spills III, modelling, analysis and control. MA: Southampton. Boston.
Giesy, J. P., Anderson, J., & Wiseman, S. B. (2010). Alberta oil sands development. Proceedings of the National Academy of Sciences of the United States of America, 107(3), 951–952.
Government of Alberta. Alberta’s oil sands resource. (2013). http://www.oilsands.alberta.ca/resource.html. Accessed June 13, 2014.
International Energy Outlook. Prepared by the Energy Information Agency, US Department of Energy. 2013. www.eia.doe.gov/oiaf/ieo/index.html. Accessed June 13, 2014.
Kelly, E. N., Schinder, W. D., Hodson, V. P., Short, W. J., Radmanovich, R., & Nielson, C. C. (2009). Oil sands development contributes polycyclic aromatic compounds to the Athabasca River and its tributaries. Proceedings of the National Academy of Sciences of the United States of America, 106(52), 22346–22351.
Kelly, E. N., Schinder, W. D., Hodson, V. P., Short, W. J., Radmanovich, R., & Nielson, C. C. (2010). Oil sands development contributes elements toxic at low concentrations to the Athabasca River and its tributaries. Proceedings of the National Academy of Sciences of the United States of America, 109(3), 4933–4937.
Kerkhoven, E., & Gan, T. Y. (2011). Unconditional uncertainties of historical and simulated river flows subjected to climate change. Journal of Hydrology, 396(1–2), 113–127.
Lanfranchi, A. L., Menone, M. L., Miglioranza, K. S. B., Janiot, L. J., Aizpu, J. E., & Moreno, V. J. (2007). Striped weakfish (Cynoscion guatucupa): a biomonitor of organo chlorine pesticides in estuarine and nearcoastal zones. Marine Pollution Bulletin, 54, 441–451.
Liang, C. P., Jang, C. S., Chen, J. S., Wang, S. W., Lee, J. J., & Liu, C. W. (2013). Probabilistic health risk assessment for ingestion of seafood farmed in arsenic contaminated groundwater in Taiwan. Environmental Geochemistry and Health, 35(4), 455–494.
Martí-Cid, R., Llobet, J. M., Castell, V., & Domingo, J. L. (2008). Evolution of the dietary exposure to polycyclic aromatic hydrocarbons in Catalonia, Spain. Food and Chemical Toxicology, 46, 3163–3171.
McGill, R., Tukey, J. W., & Larsen, W. A. (1978). Variation of box plots. American Statistician, 32, 12–16.
Muir, A. M., Sutton, T. M., Arts, M. T., Claramunt, R. M., Ebener, M. P., Fitzsimons, J. D., et al. (2010). Does condition of Lake Whitefish spawners affect physiological condition of juveniles? Journal of Great Lakes Research, 36(1), 92–99.
Nelson, J. S., & Paetz, M. J. (1992). The fishes of alberta. Calgary: The University of Calgary Press.
Nisbet, I. C. T., & LaGoy, P. K. (1992). Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regulatory Toxicology and Pharmacology, 16(3), 290–300.
Nkpaa, K. W., Wegwu, M. O., & Essien, E. B. (2013). Assessment of polycyclic aromatic hydrocarbons (PAHs) levels in two commercially important fish species from crude oil polluted waters of ogoniland and their carcinogenic health risks. Journal of Environment and Earth Science, 3(8), 128–137.
Ohiozebau, E., Tendler, B., Hill, A., Godling, G., Kelly, E., Giesy, J. P., & Jones, P. J. (2015). Products of biotransformation of polycyclic aromatic hydrocarbons in fishes of the Athabasca/Slave river system. Canada: Environ Geochem Health. doi:10.1007/s10653-015-9744-6.
Pampanin, D. M., & Sydnes, M. O. (2013). Polycyclic aromatic hydrocarbons a constituent of petroleum: Presence and influence in the aquatic environment. INTECH: Cited February, 2013, from http://creativecommons.org/licenses/3.0 .
Parajulee, A., & Wania, F. (2014). Evaluating officially reported polycyclic aromatic hydrocarbon emissions in the Athabasca oilsands region with a multimedia fate model. Proceedings of the National Academy of Sciences of the United States of America, 111(9), 3344–3349.
Pompa, G., Caloni, F., & Fracchiolla, M. L. (2003). Dioxin and PCB contamination of fish and shellfish: Assessment of human exposure: Review of the international situation. Veterinary Research Communications, 27, 159–167.
Ramalhosa, M. J., Paíga, P., Morais, S., Ramos, S., Delerue-Matos, C., & Oliveira, M. B. P. P. (2012). Polycyclic aromatic hydrocarbon levels in three pelagic fish species from Atlantic Ocean: Inter-specific and inter-season comparisons and assessment of potential public health risks. Food and Chemical Toxicology, 50(2), 162–167.
Richardson, M. G. (1997). Compedium of Canadian human exposure factors for risk assessment. Ottawa, ON: O’Connor Associates Environmental Inc.
Richardson, M. G. (2013). Canadian exposure factors handbook. Ottawa, ON: Stantec Consulting Ltd.
Rocher, V., Azimi, S., Moilleron, R., & Chebbo, G. (2004). Hydrocarbons and heavy metals in the different sewer deposits in the “Le Marais” catchment (Paris, France): Stocks, distributions and origins. Science of the Total Environment, 323, 107–122.
Schindler, D. W., Kidd, K. A., Muir, D. C. G., & Lockhart, W. L. (1995). The effects of ecosystem characteristics on contaminant distribution in northern freshwater lakes. Science of the Total Environment, 160–161, 1–17.
Scott, W. B., & Crossman, E. J. (1979). Freshwater fishes of Canada. Ottawa: The Bryant Press Limited.
Sidhu, K. S. (2003). Health benefits and potential risks related to consumption of fish or fish oil. Regulatory Toxicology and Pharmacology, 38, 336–344.
Simonin, H. A., Loukmas, J. J., Skinner, L. C., & Roy, K. M. (2008). Lake variability: Key factors controlling mercury concentrations in New York State fish. Environmental Pollution, 154(1), 107–115.
Stacewicz-Sapuntzakis, M., Borthakur, G., Burns, J. L., & Bowen, P. E. (2008). Correlations of dietary patterns with prostate health. Molecular Nutrition and Food Research, 52, 114–130.
Thyssen, J., Althoff, J., Kimmerle, G., & Mohr, U. (1981). Inhalation studies with benzo[a]pyrene in Syrian golden hamsters. Journal of National Cancer Institute, 66, 575–577.
Timilsina, G. R., LeBlanc, N. & Walden, T. (2005). Economic impacts of Alberta’s oil sands. Prepared for the Canadian Energy Research Institute. 2005. http://www.ceri.ca/docs/OilSandsReport-Final.PDF. Accessed July 15, 2015.
Timoney, K. P., & Lee, P. (2009). Does the Alberta Tar sands industry pollute? The scientific evidence. The Open Conservation Biology Journal, 3, 65–81.
Timoney, K. P., & Lee, P. (2011). Polycyclic aromatic hydrocarbons increase in Athabasca River delta sediment: Temporal trends and environmental correlates. Environmental Science and Technology, 45, 4278–4284.
USEPA. 1991a. Dose-response analysis of ingested benzo[a]pyrene (CAS No. 50-32-8). Vol. EPA/600/R-92/045. Washington, DC: Human Health Assessment Group, Office of Health and Environmental Assessment.
Usydus, Z., Szlinder-Richert, J., Polak-Juszczak, L., Komar, K., Adamczyk, M., Malesa- Ciecwierz, M., & Ruczynska, W. (2009). Fish products available in Polish market—Assessment of the nutritive value and human exposure to dioxins and other contaminants. Chemosphere, 74, 1420–1428.
Walker, C. H., Sibly, R. M., Hopkin, S. P., & Peakall, D. B. (2012). Fates of organic pollutants in individuals and organisms. Principles of ecotoxicology (pp. 63–93). New York: CRC Press.
Wei, X., Huang, Y., Wong, M. H., Giesy, J. P., & Wong, C. K. C. (2011). Assessment of risk to humans of bisphenol: A in marine and freshwater fish from Pearl River Delta. China. Chemosphere, 85(1), 122–128.
Weinhold, B. (2011). Alberta’s oil sands: Hard evidence, missing data, New Promises. Environmental Health Perspectives, 119(3), 129–131.
Wiklund, J. A., Hall, R. I., Wolfe, B. B., Edwards, T. W. D., Farwell, A. J., & Dixon, D. G. (2012). Has Alberta oil sands development increased far-field delivery of airborne contaminants to the Peace-Athabasca Delta? Science of the Total Environment, 433, 379–382.
Wretling, S., Eriksson, A., Eskhult, G. A., & Larsson, B. (2010). Polycyclic aromatic hydrocarbons (PAHs) in Swedish smoked meat and fish. Journal of Food Consumption and Analysis, 23, 264–272.
**a, Z., Duan, X., Qiu, W., Liu, D., Wang, B., Tao, S., et al. (2010). Health risk assessment on dietary exposure to polycyclic aromatic hydrocarbons (PAHs) in Taiyuan, China. Science of The Total Environment, 408(22), 5331–5337.
Yoon, E., Park, K., Lee, H., Yang, J. H., & Lee, C. (2007). Estimation of excess cancer risk on time -weighted lifetime average daily intake of PAHs from food ingestion. Human Ecological Risk Assess, 13(3), 669–680.
Acknowledgments
The authors would like to appreciate First Nations and Métis communities of Fort Resolution, Fort Smith, Fort Chipewyan, Fort McKay and Fort McMurray and numerous Provincial and Federal agencies for their assistance during the sampling. The Slave River and Delta Partnership provided invaluable assistance in the coordination of collection and assessment activities in the Slave River and Delta. Portions of this work were funded by the Boreal Songbird Initiative (BSI); Aboriginal Affairs and Northern Development Canada (AANDC); and the Government of the Northwest Territories (GNWT). EO was supported by a New Faculty Scholarship to PDJ from the University of Saskatchewan. Prof. Giesy was supported by the Canada Research Chair program and the program of 2014 ‘High Level Foreign Experts’ (#GDT20143200016) funded by the State Administration of Foreign Experts Affairs, the PR China to Nan**g University and the Einstein Professor Program of the Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
Lognormal probability density functions describing daily fish consumption (g/day) for Canadian Aboriginal fish ‘eaters only.’ Individuals reporting no fish consumption were excluded. Values were rounded to two significant digits. Values represent arithmetic mean ± standard deviation for definition of lognormal distributions. Different values for males and females are indicated only where statistically significant differences were observed between the sexes in the data. Values represent, respectively, the arithmetic mean ± standard deviation (ARITH), the arithmetic mean and standard deviation of the log-transformed data (LN-TRANS), the geometric mean and geometric standard deviation (GEOMET) (Richardson 1997, 2013).
Gender | Children | Teens | Adults | Senior |
|---|---|---|---|---|
Females | ||||
ARITH | 170 ± 150 | 150 ± 150 | 180 ± 140 | 250 ± 240 |
LN-TRANS | 4.85 ± 0.76 | 4.66 ± 0.83 | 4.96 ± 0.69 | 5.19 ± 0.81 |
GEOMET | 128 ± 2.1 | 106 ± 2.3 | 143 ± 2.0 | 179 ± 2.2 |
Males | ||||
ARITH | 170 ± 150 | 260 ± 250 | 270 ± 190 | 250 ± 240 |
LN-TRANS | 4.85 ± 0.76 | 5.23 ± 0.81 | 5.40 ± 0.63 | 5.19 ± 0.81 |
GEOMET | 128 ± 2.1 | 187 ± 2.2 | 221 ± 1.9 | 179 ± 2.2 |
Sexes combined | ||||
ARITH | 170 ± 150 | 200 ± 200 | 220 ± 160 | 250 ± 240 |
LN-TRANS | 4.85 ± 0.76 | 4.95 ± 0.83 | 5.18 ± 0.65 | 5.19 ± 0.81 |
GEOMET | 128 ± 2.1 | 141 ± 2.3 | 178 ± 1.9 | 179 ± 2.2 |
Appendix 2
Proposed probability density functions describing body weight (kg) in the Canadian population. In all cases, PDFs should be defined as lognormal. Values represent, respectively, the arithmetic mean ± standard deviation (ARITH), the arithmetic mean and standard deviation of the log-transformed data (LN-TRANS), the geometric mean and geometric standard deviation (GEOMET) (Richardson 1997, 2013).
Age group | Distribution | Females | Males | Sexes combined |
|---|---|---|---|---|
Infants (0–6 months) | Arth | 8.2 ± 2.9 | ||
Ln-Trans | – | – | 2.05 ± 0.34 | |
Geomet | – | – | 7.8 ± 1.4 | |
Toddlers (7 months–4 years) | Arth | 16.4 ± 4.5 | 16.5 ± 4.6 | 16.5 ± 4.5 |
Ln-Trans | 2.76 ± 0.27 | 2.77 ± 0.27 | 2.77 ± 0.27 | |
Geomet | 15.8 ± 1.3 | 16.0 ± 1.3 | 16.0 ± 1.3 | |
Children (5–11 years) | Arth | 33.6 ± 9.3 | 32.2 ± 8.0 | 32.9 ± 8.9 |
Ln-Trans | 3.48 ± 0.27 | 3.44 ± 0.24 | 3.46 ± 0.27 | |
Geomet | 32.5 ± 1.3 | 31.2 ± 1.3 | 31.8 ± 1.3 | |
Teens (12–19 years) | Arth | 56.2 ± 10.2 | 63.1 ± 15.3 | 59.7 ± 13.5 |
Ln-Trans | 4.01 ± 0.18 | 4.12 ± 0.24 | 4.06 ± 0.22 | |
Geomet | 55.1 ± 1.2 | 61.6 ± 1.3 | 58.0 ± 1.2 | |
Adults (20–59 years) | Arth | 63.1 ± 11.9 | 78.8 ± 12.3 | 70.7 ± 14.4 |
Ln-Trans | 4.13 ± 0.18 | 4.35 ± 0.16 | 4.24 ± 0.20 | |
Geomet | 62.2 ± 1.2 | 77.5 ± 1.2 | 69.4 ± 1.2 | |
Seniors (60+ years) | Arth | 63.4 ± 11.6 | 78.9 ± 14.2 | 70.6 ± 15.0 |
Ln-Trans | 4.13 ± 0.18 | 4.35 ± 0.18 | 4.23 ± 0.21 | |
Geomet | 62.2 ± 1.2 | 77.5 ± 1.2 | 68.7 ± 1.2 | |
Adults (20+ years) | Arth | 63.1 ± 11.8 | 78.8 ± 12.6 | 70.7 ± 14.5 |
Ln-Trans | 4.13 ± 0.19 | 4.35 ± 0.16 | 4.24 ± 0.20 | |
Geomet | 62.2 ± 1.2 | 77.5 ± 1.2 | 69.4 ± 1.2 |
Appendix 3
Mean (± SD) values for parameters, including: length (cm), mass (g)and liver somatic index (LSI) of fishes collected at Fort Resolution, Fort Smith, Fort Chipewyan, Fort McKay and Fort McMurray in 2011–2012 in (A) summer, (B) fall, (C) spring. Number of individual fish collected indicated in brackets (n). n.a = no specimen available this location/season. F = Fort.
Fish species | F. McMurray | F. McKay | F. Chipewyan | F. Smith | F. Resolution | |
|---|---|---|---|---|---|---|
3a. Summer | ||||||
Burbot | Length | 41 ± 3.4 (3) | n.a | 42 ± 3.4 (2) | 50 ± 9.2 (5) | 62 ± 4.4 (10) |
Mass | 420 ± 104(3) | n.a | 693 ± 104 (2) | 577 ± 320 (5) | 1591 ± 341 (10) | |
LSI | 6.9 ± 1.5 (3) | n.a | 5.1 ± 1.5(2) | 2.0 ± 0.2 (5) | 13 ± 21 (10) | |
Goldeye | Length | 35 ± 4.5 (10) | 38 ± 2.7 (10) | 37 ± 1.1 (10) | 29 ± 3.5 (10) | 38 ± 1.8 (2) |
Mass | 489 ± 154 (10) | 685 ± 140 (10) | 573 ± 55 (10) | 221 ± 95 (10) | 646 ± 153 (2) | |
LSI | 1.2 ± 0.3 (10) | 1.5 ± 0.2 (10) | 1.2 ± 0.3 (10) | 0.7 ± 0.2 (10) | 1.1 ± 0.1 (2) | |
Jackfish | Length (cm) | 61 ± 22 (10) | 62 ± 10 (10) | 66 ± 5.1 (10) | 68 ± 505 (10) | 64 ± 4.2 (10) |
Mass (g) | 1610 ± 1369 (10) | 1938 ± 1172 (10) | 2178 ± 1102(10) | 2457 ± 981 (10) | 1976 ± 1276 (10) | |
LSI | 1.4 ± 0.7 (10) | 1.8 ± 0.4 (10) | 0.8 ± 0.3 (10) | 1.4 ± 0.6 (10) | 3.3 ± 4.8 (10) | |
Walleye | Length | 5.8 ± 10 (10) | 45 ± 13 (10) | 51 ± 3.4 (10) | 40 ± 7.6 (10) | n.a |
Mass | 1347 ± 646 (10) | 1003 ± 566 (10) | 1365 ± 247(10) | 644 ± 364 (10) | n.a | |
LSI | 1.1 ± 0.3 (10) | 1.0 ± 0.3 (10) | 1.1 ± 0.4 (10) | 0.8 ± 0.2 (10) | n.a | |
Whitefish | Length (cm) | n.a | 42 ± 4.2(10) | 41 ± 3.4 (10) | 41.1 ± 3.7 (8) | 39 ± 1.9 (10) |
Mass | n.a | 1281 ± 323 (10) | 1177 ± 324 (10) | 864 ± 145 (8) | 685 ± 223 (10) | |
LSI | n.a | 1.0 ± 0.2 (10) | 1.2 ± 0.3 (10) | 0.8 ± 0.3 (8) | 1.9 ± 3.2 (10) | |
3b. Fall | ||||||
Burbot | Length | n.a | 55 ± 0.9 (2) | 59 ± 2.8 (3) | 61 ± 5.1 (3) | 61 ± 5.0 (10) |
Mass | n.a | 1075 ± 7.1 (2) | 1387 ± 74 (3) | 1335 ± 158 (3) | 1662 ± 404 (10) | |
LSI | n.a | 2.1 ± 0.1 (2) | 3.0 ± 0.4 (3) | 2.9 ± 0.4 (3) | 3.2 ± 1.4 (10) | |
Goldeye | Length | 39 ± 0.0 (1) | 36 ± 1.4 (10) | 37 ± 2.7 (10) | 36 ± 1.3 (10) | 36 ± 0.9 (10) |
Mass | 700 ± 0.0 (1) | 537 ± 47 (10) | 627 ± 95 (10) | 552 ± 66 (10) | 546 ± 65 (10) | |
LSI | 1.4 ± 0.0 (1) | 1.3 ± 0.1 (10) | 1.5 ± 0.5 (10) | 2.1 ± 3.1 (10) | 1.3 ± 0.2 (10) | |
Jackfish | Length | 72 ± 14 (3) | 63 ± 8.8 (9) | 76 ± 2.5 (10) | 67 ± 8.4 (10) | 69 ± 11 (10) |
Mass | 3287 ± 1454(3) | 2531 ± 1415 (9) | 4220 ± 1157 (10) | 1390 ± 522 (10) | 1266 ± 538 (10) | |
LSI | 1.9 ± 0.4 (3) | 1.9 ± 0.3 (9) | 1.7 ± 0.2 (10) | 1.1 ± 0.5 (10) | 1.2 ± 0.4 (10) | |
Walleye | Length | 42 ± 11 (3) | 49 ± 4.8 (10) | 50 ± 2.5 (5) | 49 ± 5.5 (10) | 47 ± 6.9 (10) |
Mass | 940 ± 588(3) | 1356 ± 408 (10) | 4220 ± 1157 (5) | 1390 ± 522 (10) | 1266 ± 538 (10) | |
LSI | 1.9 ± 0.1 (3) | 1.5 ± 0.5 (10) | 1.7 ± 0.2 (5) | 1.3 ± 0.4 (10) | 2.4 ± 1.2 (10) | |
Whitefish | Length | 42 ± 3.4 (10) | 40 ± 2.2 (10) | 39 ± 3.1 (10) | 41 ± 1.8 (10) | 44 ± 3.5 (10) |
Mass | 1042 ± 235 (10) | 1020 ± 150 (10) | 1072 ± 200 (10) | 1019 ± 125(10) | 1296 ± 38 (10) | |
LSI | 0.8 ± 0.1 (10) | 0.8 ± 0.2 (10) | 1.4 ± 0.4 (10) | 0.8 ± 0.2 (10) | 0.9 ± 0.2 (10) | |
3c. Spring | ||||||
Burbot | Length | 39 ± 2.6 (3) | n.a | n.a | 38 ± 0.0 (1) | 63 ± 3.3 (6) |
Mass | 420 ± 87 (3) | n.a | n.a | 750 ± 0.0 (1) | 1623 ± 632 (6) | |
LSI | 5.2 ± 1.9 (3) | n.a | n.a | 1.1 ± 0.0 (1) | 7.5 ± 3.7 (6) | |
Goldeye | Length | 34 ± 2.9 (10) | 27 ± 5.1 (10) | 35 ± 3.1 (10) | 37 ± 1.9 (10) | 35 ± 3.8 (10) |
Mass | 524 ± 113 (10) | 285 ± 186 (10) | 490 ± 109 (10) | 570 ± 100(10) | 554 ± 166 (10) | |
LSI | 1.1 ± 0.2 (10) | 1.4 ± 0.2 (10) | 1.5 ± 0.6 (10) | 1.3 ± 0.2 (10) | 1.3 ± 0.2 (10) | |
Jackfish | Length | 63 ± 9.1 (10) | 60 ± 7.2 (5) | 63 ± 8.0 (10) | 69 ± 11 (10) | 69 ± 5.8 (10) |
Mass | 3389 ± 1209 (10) | 1862 ± 1425 (5) | 1653 ± 468. (10) | 3237 ± 1508 (10) | 2272 ± 1020 (10) | |
LSI | 1.7 ± 0.6 (10) | 1.4 ± 0.5 (5) | 1.2 ± 0.5 (10) | 1.4 ± 0.2 (10) | 2.6 ± 4.4 (10) | |
Walleye | Length | 48 ± 6.8 (10) | 44 ± 2.6 (10) | 50 ± 6.6 (10) | 51 ± 8.7 (10) | 46 ± 13 (10) |
Mass | 1740 ± 870 (10) | 1092 ± 148(10) | 1367 ± 398 (10) | 1623 ± 771 (10) | 1180 ± 712 (10) | |
LSI | 1.2 ± 0.4 (10) | 1.2 ± 0.3 (10) | 1.4 ± 0.3 (10) | 1.6 ± 0.5 (10) | 1.5 ± 0.4 (10) | |
Whitefish | Length | 42 ± 2.0 (4) | 38 ± 1.8 (2) | 43 ± 5.8 (10) | 41 ± 1.3 (5) | 39 ± 2.6 (10) |
Mass | 1278 ± 315 (4) | 1025 ± 35(2) | 1384 ± 392 (10) | 990 ± 115.3 (5) | 807 ± 197 (10) | |
LSI | 1.2 ± 0.1 (4) | 1.0 ± 0.0 (2) | 1.3 ± 0.2 (10) | 0.9 ± 0.2 (5) | 1.1 ± 0.3 (10) | |
Rights and permissions
About this article
Cite this article
Ohiozebau, E., Tendler, B., Codling, G. et al. Potential health risks posed by polycyclic aromatic hydrocarbons in muscle tissues of fishes from the Athabasca and Slave Rivers, Canada. Environ Geochem Health 39, 139–160 (2017). https://doi.org/10.1007/s10653-016-9815-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-016-9815-3