Abstract
Background and Aims
Inflammatory bowel disease (IBD) is currently gaining an increasing global interest. Intestinal epithelial barrier dysfunction is crucial toward develo** IBD; however, the underlying mechanisms are not yet elucidated. This study is aimed at elucidating the function of CRL4DCAF2, an E3 ligase, toward mediating intestinal homeostasis.
Methods
Colon samples were collected from patients with IBD and healthy individuals to examine the expression of CRL4DCAF2. CRL4DCAF2 conditional knockdown in mouse intestinal epithelial cells (IECs) (DCAF2EKD) were constructed. DCAF2EKD and their littermate control (DCAF2EWT) were treated with dextran sodium sulfate (DSS) to induce acute colitis. Transcriptome analysis was performed on inflamed colon samples obtained from the mice. Cell cycle regulators were evaluated using real-time polymerase chain reaction (PCR), while tight junction and apoptosis proteins were examined via immunofluorescence and western blot.
Results
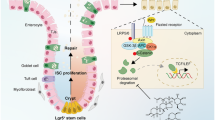
CRL4DCAF2 expression was significantly decreased in the inflamed IBD epithelium, and low expression of CRL4DCAF2 associated with high recurrence risk. Mice with DCAF2 specific knockout in IECs suffer from embryonic death. Multiple genes involved in cell proliferation, immune response, and gap junction were differentially expressed in inflamed colon from DCAF2EKD compared with DCAF2EWT. Furthermore, conditional downregulation of CRL4DCAF2 in the intestinal epithelium induced primarily epithelial damage, increased intestinal permeability, and diminished tight junction protein expression. In vivo and in vitro cell transfection experiments revealed that CRL4DCAF2 enhanced cell proliferation by promoting p21 ubiquitination and degradation, thereby inhibiting G2/M cell cycle. In addition, CRL4DCAF2 can also inhibit IEC apoptosis and promote cell autophagy.
Conclusions
CRL4DCAF2 downregulation in IECs promotes intestinal barrier dysfunction and inhibits IEC proliferation, thus making it more susceptible to inflammation.









Similar content being viewed by others
Data availability
Sequence data that support the findings of this study are available from the authors and have been deposited in the National Center for Biotechnology Information (NCBI) BioProject with the primary accession code PRJNA791130.
References
Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol 2012;9:599–608.
Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol 2010;28:573–621.
Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 2017;152:313–321.
Molodecky NA, Soon IS, Rabi DM et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142:46–54.
Kobayashi T, Siegmund B, Le Berre C et al. Ulcerative colitis. Nat Rev Dis Primers 2020;6:74.
Roda G, Chien Ng S, Kotze PG et al. Crohn’s disease. Nat Rev Dis Primers 2020;6:22.
Scarpa M, Stylianou E. Epigenetics: concepts and relevance to IBD pathogenesis. Inflamm Bowel Dis 2012;18:1982–1996.
Blair SA, Kane SV, Clayburgh DR, Turner JR. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab Invest 2006;86:191–201.
Heller F, Florian P, Bojarski C et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 2005;129:550–564.
Zeissig S, Burgel N, Gunzel D et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 2007;56:61–72.
Abbas T, Sivaprasad U, Terai K, Amador V, Pagano M, Dutta A. PCNA-dependent regulation of p21 ubiquitylation and degradation via the CRL4Cdt2 ubiquitin ligase complex. Genes Dev 2008;22:2496–2506.
Sansam CL, Shepard JL, Lai K et al. DTL/CDT2 is essential for both CDT1 regulation and the early G2/M checkpoint. Genes Dev 2006;20:3117–3129.
Terai K, Abbas T, Jazaeri AA, Dutta A. CRL4(Cdt2) E3 ubiquitin ligase monoubiquitinates PCNA to promote translesion DNA synthesis. Mol Cell 2010;37:143–149.
Fan K, Wang F, Li Y et al. CRL4(DCAF2) is required for mature T-cell expansion via Aurora B-regulated proteasome activity. J Autoimmun 2019;96:74–85.
Huang T, Gao Z, Zhang Y et al. CRL4(DCAF2) negatively regulates IL-23 production in dendritic cells and limits the development of psoriasis. J Exp Med 2018;215:1999–2017.
Yu JS, Huang T, Zhang Y et al. Substrate-specific recognition of IKKs mediated by USP16 facilitates autoimmune inflammation. Sci Adv 2021;7:eabc4009.
Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 1993;69:238–249.
Panagopoulos A, Taraviras S, Nishitani H, Lygerou Z. CRL4(Cdt2): coupling genome stability to ubiquitination. Trends Cell Biol 2020;30:290–302.
Hou L, Zhao J, Gao S et al. Restriction of hepatitis B virus replication by c-Abl-induced proteasomal degradation of the viral polymerase. Sci Adv 2019;5:eaau7130.
Benamar M, Guessous F, Du K et al. Inactivation of the CRL4-CDT2-SET8/p21 ubiquitylation and degradation axis underlies the therapeutic efficacy of pevonedistat in melanoma. EBioMedicine 2016;10:85–100.
Champeris Tsaniras S, Kanellakis N, Symeonidou IE, Nikolopoulou P, Lygerou Z, Taraviras S. Licensing of DNA replication, cancer, pluripotency and differentiation: an interlinked world? Semin Cell Dev Biol 2014;30:174–180.
Liontos M, Koutsami M, Sideridou M et al. Deregulated overexpression of hCdt1 and hCdc6 promotes malignant behavior. Cancer Res 2007;67:10899–10909.
Xu YW, Cao LR, Wang M et al. Maternal DCAF2 is crucial for maintenance of genome stability during the first cell cycle in mice. J Cell Sci 2017;130:3297–3307.
Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 2014;14:141–153.
Bhat AA, Uppada S, Achkar IW et al. Tight junction proteins and signaling pathways in cancer and inflammation: a functional crosstalk. Front Physiol 1942;2018:9.
Laukoetter MG, Nava P, Lee WY et al. JAM-A regulates permeability and inflammation in the intestine in vivo. J Exp Med 2007;204:3067–3076.
Vetrano S, Rescigno M, Cera MR et al. Unique role of junctional adhesion molecule-a in maintaining mucosal homeostasis in inflammatory bowel disease. Gastroenterology 2008;135:173–184.
Blander JM. Death in the intestinal epithelium-basic biology and implications for inflammatory bowel disease. FEBS J 2016;283:2720–2730.
Blander JM. On cell death in the intestinal epithelium and its impact on gut homeostasis. Curr Opin Gastroenterol 2018;34:413–419.
van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 2009;71:241–260.
Gunther C, Neumann H, Neurath MF, Becker C. Apoptosis, necrosis and necroptosis: cell death regulation in the intestinal epithelium. Gut 2013;62:1062–1071.
Havens CG, Walter JC. Mechanism of CRL4(Cdt2), a PCNA-dependent E3 ubiquitin ligase. Genes Dev 2011;25:1568–1582.
Iwamoto M, Koji T, Makiyama K, Kobayashi N, Nakane PK. Apoptosis of crypt epithelial cells in ulcerative colitis. J Pathol 1996;180:152–159.
Dannappel M, Vlantis K, Kumari S et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature 2014;513:90–94.
Gunther C, Martini E, Wittkopf N et al. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature 2011;477:335–339.
Scherr AL, Gdynia G, Salou M et al. Bcl-xL is an oncogenic driver in colorectal cancer. Cell Death Dis 2016;7:e2342.
van der Heijden M, Zimberlin CD, Nicholson AM et al. Bcl-2 is a critical mediator of intestinal transformation. Nat Commun 2016;7:10916.
Welz PS, Wullaert A, Vlantis K et al. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature 2011;477:330–334.
Zhou P, Yan F. CRL4 ubiquitin pathway and DNA damage response. Adv Exp Med Biol 2020;1217:225–239.
Patankar JV, Becker C. Cell death in the gut epithelium and implications for chronic inflammation. Nat Rev Gastroenterol Hepatol 2020;17:543–556.
Wu Y, Tang L, Wang B, Sun Q, Zhao P, Li W. The role of autophagy in maintaining intestinal mucosal barrier. J Cell Physiol 2019;234:19406–19419.
Acknowledgments
We thank the Life Sciences Institute core facilities, Zhejiang University, for technical assistance. We also thank Prof. ** ** for the mice and expression plasmids. This study was supported by the Zhejiang Provincial Natural Science Foundation of China (LQ21H030010 and LQ21H030013), the National Natural Science Foundation of China (32000628), and the Zhejiang Province Medicine and Health Research Project (2021417815, 2021KY104 and 2023KY804)
Funding
This study was supported by the Zhejiang Provincial Natural Science Foundation of China (LQ21H030010andLQ21H030013), the National Natural Science Foundation of China (32000628), and the Zhejiang Province Medicine and Health Research Project (2021417815, 2021KY104, and 2023KY804).
Author information
Authors and Affiliations
Contributions
YZ and QC were responsible for conceptualization; YZ, CW, and CB for methodology; KH, LW, LY, ZZ, and RL for investigation; YZ, CW, LW, and QC for writing: original draft; YZ, CW, and QC for writing: review and editing; YZ, CW, and XG for visualization; YZ and QC for supervision; and YZ, RL, MX, and QC for funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval and consent to participate
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of Sir Run Run Shaw Hospital (SRRSH) (ID: 2022–0369). The patients provided their written informed consent to participate in this study. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Declaration of Helsinki and its later amendments. All working stages with animals were conducted following the International Guiding Principles for Biomedical Research Involving Animals in 1985. The study protocol was adhered to (Animal Research: Reporting of In Vivo Experiments (ARRIVE) guideline. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
An invited commentary on this article is available at https://doi.org/10.1007/s10620-023-08148-0.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Wang, C., Wu, L. et al. Epithelial CRL4DCAF2 Is Critical for Maintaining Intestinal Homeostasis Against DSS-Induced Colitis by Regulating the Proliferation and Repair of Intestinal Epithelial Cells. Dig Dis Sci 69, 66–80 (2024). https://doi.org/10.1007/s10620-023-08147-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-08147-1