Abstract
Background
Although arsenic trioxide (ATO) is used in the treatment of advanced hepatocellular carcinoma (HCC) in clinical trials, it is not satisfactory in terms of improving HCC patients’ overall survival. Intratumoral hypoxia and overexpression of hypoxia-inducible factor-1α (HIF-1α) may result in ATO resistance and tumor progression.
Aims
We investigated the mechanisms involving HIF-1α expression and acquired ATO chemoresistance in HCC cells and mice.
Methods
The therapeutic effects of ATO in normoxic and hypoxic HCC cells were assessed using cell viability and apoptosis assays in vitro and a xenograft model in vivo. mRNA and protein expression of HIF-1α, P-glycoprotein, and VEGF were measured by qRT-PCR and western blotting. HIF-1α inhibition was performed to investigate the mechanism of ATO resistance. VEGF secretion was tested using ELISA and tube formation assays.
Results
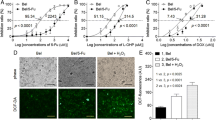
Compared to normoxic cells, hypoxic HCC cells were more resistant to ATO, with higher IC50 values and less apoptosis, and upregulated HIF-1α protein expression, accompanied with the enhancement of P-glycoprotein and VEGF synthesis after ATO treatment. VEGF secretion was elevated in the supernatant of ATO-treated HCC cells, and this change can potentiate angiogenesis in vitro. HIF-1α inhibition attenuated ATO resistance and angiogenesis and promoted the anticancer effects of ATO both in vitro and in vivo by downregulating therapy-induced P-glycoprotein and VEGF overexpression.
Conclusions
Hypoxic HCC cells acquire ATO resistance by upregulating HIF-1α levels; thus, combining ATO with a HIF-1α-targeting agent may lead to enhanced antitumor effects in HCC.






Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its additional information files.
References
Rimassa L, Assenat E, Peck-Radosavljevic M et al. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): a final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol. 2018;19:682–693.
Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15:599–616.
Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology 2016;150:835–853.
Bruix J, Takayama T, Mazzaferro V et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344–1354.
Llovet JM, Ricci S, Mazzaferro V et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390.
Liang Y, Zheng T, Song R et al. Hypoxia-mediated sorafenib resistance can be overcome by EF24 through Von Hippel–Lindau tumor suppressor-dependent HIF-1alpha inhibition in hepatocellular carcinoma. Hepatology. 2013;57:1847–1857.
Kudo M, Finn RS, Qin S et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163–1173.
Zhang XW, Yan XJ, Zhou ZR et al. Arsenic trioxide controls the fate of the PML-RARalpha oncoprotein by directly binding PML. Science 2010;328:240–243.
Valenzuela M, Glorieux C, Stockis J et al. Retinoic acid synergizes ATO-mediated cytotoxicity by precluding Nrf2 activity in AML cells. Br J Cancer 2014;111:874–882.
Platanias LC. Biological responses to arsenic compounds. J Biol Chem. 2009;284:18583–18587.
Roboz GJ, Dias S, Lam G et al. Arsenic trioxide induces dose- and time-dependent apoptosis of endothelium and may exert an antileukemic effect via inhibition of angiogenesis. Blood. 2000;96:1525–1530.
Zhang X, Yang XR, Sun C et al. Promyelocytic leukemia protein induces arsenic trioxide resistance through regulation of aldehyde dehydrogenase 3 family member A1 in hepatocellular carcinoma. Cancer Lett. 2015;366:112–122.
Lin CC, Hsu C, Hsu CH, Hsu WL, Cheng AL, Yang CH. Arsenic trioxide in patients with hepatocellular carcinoma: a phase II trial. Investig New Drugs. 2007;25:77–84.
Subbarayan PR, Ardalan B. In the war against solid tumors arsenic trioxide needs partners. J Gastrointest Cancer 2014;45:363–371.
Zheng T, Yin D, Lu Z et al. Nutlin-3 overcomes arsenic trioxide resistance and tumor metastasis mediated by mutant p53 in Hepatocellular carcinoma. Mol Cancer. 2014;13:133.
Tredan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99:1441–1454.
Worns MA, Galle PR. HCC therapies–lessons learned. Nat Rev Gastroenterol Hepatol. 2014;11:447–452.
Wilson GK, Tennant DA, McKeating JA. Hypoxia inducible factors in liver disease and hepatocellular carcinoma: current understanding and future directions. J Hepatol. 2014;61:1397–1406.
Liu LP, Hu BG, Ye C, Ho RL, Chen GG, Lai PB. HBx mutants differentially affect the activation of hypoxia-inducible factor-1alpha in hepatocellular carcinoma. Br J Cancer. 2014;110:1066–1073.
Cao S, Yang S, Wu C, Wang Y, Jiang J, Lu Z. Protein expression of hypoxia-inducible factor-1 alpha and hepatocellular carcinoma: a systematic review with meta-analysis. Clin. Res Hepatol Gastroenterol. 2014;38:598–603.
Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer 2003;3:721–732.
Paez-Ribes M, Allen E, Hudock J et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer cell. 2009;15:220–231.
Welti J, Loges S, Dimmeler S, Carmeliet P. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J Clin Investig. 2013;123:3190–3200.
Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13:871–882.
Yeo EJ, Chun YS, Cho YS et al. YC-1: a potential anticancer drug targeting hypoxia-inducible factor 1. J Natl Cancer Inst. 2003;95:516–525.
Wu C, Gong F, Pang P, et al. An RGD-modified MRI-visible polymeric vector for targeted siRNA delivery to hepatocellular carcinoma in nude mice. PloS One. 2013;8:e66416.
Tung JN, Cheng YW, Hsu CH et al. Normoxically overexpressed hypoxia inducible factor 1-alpha is involved in arsenic trioxide resistance acquisition in hepatocellular carcinoma. Ann Surg Oncol. 2011;18:1492–1500.
Liu B, Pan S, Dong X et al. Opposing effects of arsenic trioxide on hepatocellular carcinomas in mice. Cancer Sci 2006;97:675–681.
Shaked Y, Ciarrocchi A, Franco M et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science 2006;313:1785–1787.
Emadi A, Gore SD. Arsenic trioxide—An old drug rediscovered. Blood Rev. 2010;24:191–199.
Maeda H, Hori S, Nishitoh H et al. Tumor growth inhibition by arsenic trioxide (As2O3) in the orthotopic metastasis model of androgen-independent prostate cancer. Cancer Res 2001;61:5432–5440.
Ahn RW, Chen F, Chen H et al. A novel nanoparticulate formulation of arsenic trioxide with enhanced therapeutic efficacy in a murine model of breast cancer. Clin Cancer Res. 2010;16:3607–3617.
Yu G, Chen X, Chen S, Ye W, Hou K, Liang M. Arsenic trioxide reduces chemo-resistance to 5-fluorouracil and cisplatin in HBx-HepG2 cells via complex mechanisms. Cancer Cell Int. 2015;15:116.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81620108017 to HS, No. 81771879 to DL), the Natural Science Foundation of Guangdong Province (No. 2017A030313807 to YC).
Author information
Authors and Affiliations
Contributions
YC, DL and HS designed the experiments. YC, HL, DC, XJ and WW performed the experiments and data analysis. YC, HL, and DC wrote the manuscript. YC, HL, DC, DL and SH critically reviewed and revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, Y., Li, H., Chen, D. et al. Hypoxic Hepatocellular Carcinoma Cells Acquire Arsenic Trioxide Resistance by Upregulating HIF-1α Expression. Dig Dis Sci 67, 3806–3816 (2022). https://doi.org/10.1007/s10620-021-07202-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-021-07202-z