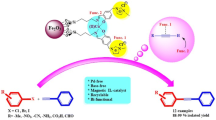
Abstract
A new versatile and recyclable NHC ligand precursor has been developed with ligand, base, and solvent functionalities for the efficient Pd-catalyzed Heck, Suzuki and Sonogashira cross-coupling reactions under mild conditions. Furthermore, NHC ligand precursor was immobilized on magnetite and its catalytic activity was also evaluated towards the coupling reactions as a heterogeneous catalyst. The NHC ligand precursor was prepared with imidazolium functionalization of TCT followed by a simple ion exchange by hydroxide ions. However, the results revealed an excellent catalytic activity for the both homogeneous and heterogeneous catalytic systems. 1.52 g.cm−3 and 1194 cP was obtained for the density and viscosity of the NHC ligand precursor respectively. On the other hand, the heterogeneous type could be readily recovered from the reaction mixture and reused for several times while preserving its properties. Heterogeneous nature of the magnetic catalyst was studied by hot filtration, mercury poisoning, and three-phase tests. High to excellent yields were obtained for all entries for the both homogeneous and heterogeneous catalysts, which reflects the high consistency of the catalyst.
Graphic Abstract













Similar content being viewed by others
References
Cheng S, Wei W, Zhang X, Yu H, Huang M, Kazemnejadi M (2020) Green Chem 22:2069–2076
Biajoli AF, Schwalm CS, Limberger J, Claudino TS, Monteiro AL (2014) J Braz Chem Soc 25:2186–2214
Yousaf M, Zahoor AF, Akhtar R, Ahmad M, Naheed S (2019) Mol Diversity 24:821–839
Asadi S, Sedghi R, Heravi MM (2017) Catal Lett 147:2045–2056
Dalby A, Mo X, Stoa R, Wroblewski N, Zhang Z, Hagen TJ (2013) Tetrahedron Lett 54:2737–2739
Thiel OR, Achmatowicz M, Milburn RM (2012) Synlett 23:1564–1574
Liu C, Liu G, Zhao H (2016) Chin J Chem 34:1048–1052
Sardarian AR, Kazemnejadi M, Esmaeilpour M (2019) Dalton Trans 48:3132–3145
Tsoukala A, Bjørsvik H-R (2011) Org Process Res Dev 15:673–680
Fu Y, Hong S, Li D, Liu S (2013) J Agric Food Chem 61:5347–5352
Nishimura K, Kinugawa M (2012) Org Process Res Dev 16:225–231
Rivara S, Piersanti G, Bartoccini F, Diamantini G, Pala D, Riccioni T, Stasi MA, Cabri W, Borsini F, Mor M, Tarzia G, Minetti P (2013) J Med Chem 56:1247–1261
Chekal BP, Guinness SM, Lillie BM, McLaughlin RW, Palmer CW, Post RJ, Sieser JE, Singer RA, Sluggett GW, Vaidyanathan R, Withbroe GJ (2014) Org Process Res Dev 18:266–274
Nasseri MA, Alavi SA, Kazemnejadi M, Allahresani A (2019) RSC Adv 9:20749–20759
Nasseri MA, Rezazadeh Z, Kazemnejadi M, Allahresani A (2019) J Iran Chem Soc 16:2693–2705
Sperry JB, Farr RM, Levent M, Ghosh M, Hoagland SM, Varsolona RJ, Sutherland K (2012) Org Process Res Dev 16:1854–1860
Loubidi M, Moutardier A, Campos JF, Berteina-Raboin S (2018) Tetrahedron Lett 59:1050–1054
Zhang ZM, Xu B, Wu L, Wu Y, Qian Y, Zhou L, Liu Y, Zhang J (2019) Angew Chem 131:14795–14801
Yim JCH, Nambo M, Crudden CM (2017) Org Lett 19:3715–3718
Laffoon SD, Chan VS, Fickes MG, Kotecki B, Ickes AR, Henle J, Napolitano JG, Franczyk TS, Dunn TB, Barnes DM, Haight AR (2019) ACS Catal 9:11691–11708
Naeimi H, Kiani F (2018) J Coord Chem 71:1157–1167
Sadjadi S, Lazzara G, Malmir M, Heravi MM (2018) J Catal 366:245–257
Chen MT, Hsieh BY, Liu YH, Wu KH, Lussari N, Braga AA (2020). Appl Organomet Chem. https://doi.org/10.1002/aoc.5870
Ghotbinejad M, Khosropour AR, Mohammadpoor-Baltork I, Moghadam M, Tangestaninejad S, Mirkhani V (2014) J Mol Catal A: Chem 385:78–84
Wang T, Xu K, Wang W, Liu L (2018) Transition Met Chem 43:347–353
Aktaş A, Celepci DB, Gök Y (2019) J Chem Sci 131:78
Andrade GA, DiMeglio JL, Guardino ET, Yap GP, Rosenthal J (2017) Polyhedron 135:134–143
Wang X, Li S, Yu H, Yu J, Liu S (2011). Chem–Eur J. https://doi.org/10.1002/chem.201101032
Kazemnejadi M, Alavi SA, Rezazadeh Z, Nasseri MA, Allahresani A, Esmaeilpour M (2019) Green Chem 21:1718–1734
Nasseri MA, Alavi SA, Kazemnejadi M, Allahresani A (2019) ChemistrySelect 4:8493–8499
Long Y, Liang K, Niu J, Tong X, Yuan B, Ma J (2015) New J Chem 39:2988–2996
Veisi H, Najafi S, Hemmati S (2018) Int J Biol Macromol 113:186–194
Kazemnejadi M, Alavi SA, Rezazadeh Z, Nasseri MA, Allahresani A, Esmaeilpour M (2019) J Mol Struct 1186:230–249
Yang Q, Sane N, Klosowski D, Lee M, Rosenthal T, Wang NX, Wiensch E (2019) Org Process Res Dev 23:2148–2156
Brown RW, Zamani F, Gardiner MG, Yu H, Pyne SG, Hyland CJ (2019) Chem Sci 10:9051–9056
Li J, Yang S, Wu W, Jiang H (2018) Eur J Org Chem 2018:1284–1306
Christoffel F, Ward TR (2018) Catal Lett 148:489–511
Acknowledgement
This work was sponsored in part by Natural Science Foundation of Liaoning Province (No. 20170520296).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Min, Q., Miao, P., Chu, D. et al. Introduction of a Recyclable Basic Ionic Solvent with Bis-(NHC) Ligand Property and The Possibility of Immobilization on Magnetite for Ligand- and Base-Free Pd-Catalyzed Heck, Suzuki and Sonogashira Cross-Coupling Reactions in Water. Catal Lett 151, 3030–3047 (2021). https://doi.org/10.1007/s10562-021-03552-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-021-03552-5