Abstract
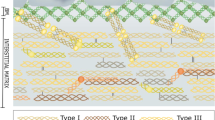
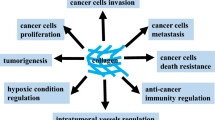
Progression through dissemination to tumor-surrounding tissues and metastasis development is a hallmark of cancer that requires continuous cell-to-cell interactions and tissue remodeling. In fact, metastization can be regarded as a tissue disease orchestrated by cancer cells, leading to neoplastic colonization of new organs. Collagen is a major component of the extracellular matrix (ECM), and increasing evidence suggests that it has an important role in cancer progression and metastasis. Desmoplasia and collagen biomarkers have been associated with relapse and death in cancer patients. Despite the increasing interest in ECM and in the desmoplastic process in tumor microenvironment as prognostic factors and therapeutic targets in cancer, further research is required for a better understanding of these aspects of cancer biology. In this review, published evidence correlating collagen with cancer prognosis is retrieved and analyzed, and the role of collagen and its fragments in cancer pathophysiology is discussed.

Similar content being viewed by others
References
Frantz, C., Stewart, K. M., & Weaver, V. M. (2010). The extracellular matrix at a glance. Journal of Cell Science, 123(24), 4195–4200. https://doi.org/10.1242/jcs.023820.
Gumbiner, B. M. (1996). Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell, 84(3), 345–357. https://doi.org/10.1016/s0092-8674(00)81279-9.
Katsumi, A., Orr, A. W., Tzima, E., & Schwartz, M. A. (2004). Integrins in mechanotransduction. The Journal of Biological Chemistry, 279(13), 12001–12004. https://doi.org/10.1074/jbc.R300038200.
Engler, A. J., Sen, S., Sweeney, H. L., & Discher, D. E. (2006). Matrix elasticity directs stem cell lineage specification. Cell, 126(4), 677–689. https://doi.org/10.1016/j.cell.2006.06.044.
Walker, C., Mojares, E., & del Río Hernández, A. (2018). Role of extracellular matrix in development and cancer progression. International Journal of Molecular Sciences, 19(10). https://doi.org/10.3390/ijms19103028.
Ricard-Blum, S. (2011). The collagen family. Cold Spring Harbor Perspectives in Biology, 3(1). https://doi.org/10.1101/cshperspect.a004978.
Kadler, K. E., Holmes, D. F., Trotter, J. A., & Chapman, J. A. (1996). Collagen fibril formation. Biochemical Journal, 316(Pt 1), 1–11.
Hashmi, S., & Marinkovich, M. P. (2011). Molecular organization of the basement membrane zone. Clinics in Dermatology, 29(4), 398–411. https://doi.org/10.1016/j.clindermatol.2011.01.009.
Kadler, K. E., Baldock, C., Bella, J., & Boot-Handford, R. P. (2007). Collagens at a glance. Journal of Cell Science, 120(12), 1955–1958. https://doi.org/10.1242/jcs.03453.
Shoulders, M. D., & Raines, R. T. (2009). Collagen structure and stability. Annual Review of Biochemistry, 78, 929–958. https://doi.org/10.1146/annurev.biochem.77.032207.120833.
Bella, J., Eaton, M., Brodsky, B., & Berman, H. M. (1994). Crystal and molecular structure of a collagen-like peptide at 1.9 A resolution. Science (New York, N.Y.), 266(5182), 75–81. https://doi.org/10.1126/science.7695699.
Persikov, A. V., Ramshaw, J. A. M., Kirkpatrick, A., & Brodsky, B. (2005). Electrostatic interactions involving lysine make major contributions to collagen triple-helix stability. Biochemistry, 44(5), 1414–1422. https://doi.org/10.1021/bi048216r.
Nimni, M. E. (1983). Collagen: structure, function, and metabolism in normal and fibrotic tissues. Seminars in Arthritis and Rheumatism, 13(1), 1–86. https://doi.org/10.1016/0049-0172(83)90024-0.
Qi, Y., & Xu, R. (2018). Roles of PLODs in collagen synthesis and cancer progression. Frontiers in Cell and Development Biology, 6. https://doi.org/10.3389/fcell.2018.00066.
Myllyharju, J. (2005). Intracellular post-translational modifications of collagens. In J. Brinckmann, H. Notbohm, & P. K. Müller (Eds.), Collagen (Vol. 247, pp. 115–147). Berlin, Heidelberg: Springer Berlin Heidelberg. https://doi.org/10.1007/b103821.
Makareeva, E., & Leikin, S. (2007). Procollagen triple helix assembly: an unconventional chaperone-assisted folding paradigm. PLoS One, 2, 403–434. https://doi.org/10.1371/journal.pone.0001029.
Martinek, N., Shahab, J., Sodek, J., & Ringuette, M. (2007). Is SPARC an evolutionarily conserved collagen chaperone? Journal of Dental Research, 86(4), 296–305. https://doi.org/10.1177/154405910708600402.
Giudici, C., Raynal, N., Wiedemann, H., Cabral, W. A., Marini, J. C., Timpl, R., et al. (2008). Map** of SPARC/BM-40/osteonectin-binding sites on fibrillar collagens. The Journal of Biological Chemistry, 283(28), 19551–19560. https://doi.org/10.1074/jbc.M710001200.
Morrissey, M. A., Jayadev, R., Miley, G. R., Blebea, C. A., Chi, Q., Ihara, S., & Sherwood, D. R. (2016). SPARC promotes cell invasion in vivo by decreasing type IV collagen levels in the basement membrane. PLoS Genetics, 12(2), e1005905. https://doi.org/10.1371/journal.pgen.1005905.
Karsdal, M. A. (2016). Introduction. In M. A. Karsdal (Ed.), Biochemistry of collagens, laminins and elastin (pp. xix–xxxiv). Academic Press. https://doi.org/10.1016/B978-0-12-809847-9.02001-8.
Canty, E. G. (2005). Procollagen trafficking, processing and fibrillogenesis. Journal of Cell Science, 118(7), 1341–1353. https://doi.org/10.1242/jcs.01731.
Hopkins, D. R., Keles, S., & Greenspan, D. S. (2007). The bone morphogenetic protein 1/tolloid-like metalloproteinases. Matrix biology : journal of the International Society for Matrix Biology, 26(7), 508–523. https://doi.org/10.1016/j.matbio.2007.05.004.
Imamura, Y., Steiglitz, B. M., & Greenspan, D. S. (1998). Bone morphogenetic protein-1 processes the NH2-terminal propeptide, and a furin-like proprotein convertase processes the COOH-terminal propeptide of pro-alpha1(V) collagen. The Journal of Biological Chemistry, 273(42), 27511–27517. https://doi.org/10.1074/jbc.273.42.27511.
Fukae, M., & Mechanic, G. L. (1980). Maturation of collagenous tissue. Temporal sequence of formation of peptidyl lysine-derived cross-linking aldehydes and cross-links in collagen. Journal of Biological Chemistry, 255(13), 6511–6518.
Kuczek, D. E., Hübbe, M. L., & Madsen, D. H. (2017). Internalization of collagen: an important matrix turnover pathway in cancer. In R. A. Brekken & D. Stupack (Eds.), Extracellular matrix in tumor biology (pp. 17–38). Cham: Springer International Publishing. https://doi.org/10.1007/978-3-319-60907-2_2.
Perumal, S., Antipova, O., & Orgel, J. P. R. O. (2008). Collagen fibril architecture, domain organization, and triple-helical conformation govern its proteolysis. Proceedings of the National Academy of Sciences, 105(8), 2824–2829. https://doi.org/10.1073/pnas.0710588105.
Lee, M., Fridman, R., & Mobashery, S. (2004). Extracellular proteases as targets for treatment of cancer metastases. Chemical Society Reviews, 33(7), 401. https://doi.org/10.1039/b209224g.
DeClerck, Y. A. (2012). Desmoplasia: a response or a niche? Cancer Discovery, 2(9), 772–774. https://doi.org/10.1158/2159-8290.CD-12-0348.
Barsky, S. H., Green, W. R., Grotendorst, G. R., & Liotta, L. A. (1984). Desmoplastic breast carcinoma as a source of human myofibroblasts. The American Journal of Pathology, 115(3), 329–333.
Wolfe, J. N. (1976). Breast patterns as an index of risk for develo** breast cancer. AJR. American Journal of Roentgenology, 126(6), 1130–1137. https://doi.org/10.2214/ajr.126.6.1130.
Guo, Y. P., Martin, L. J., Hanna, W., Banerjee, D., Miller, N., Fishell, E., et al. (2001). Growth factors and stromal matrix proteins associated with mammographic densities. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 10(3), 243–248.
Boyd, N. F., Guo, H., Martin, L. J., Sun, L., Stone, J., Fishell, E., et al. (2007). Mammographic density and the risk and detection of breast cancer. New England Journal of Medicine, 356(3), 227–236. https://doi.org/10.1056/NEJMoa062790.
Ayala, G., Tuxhorn, J. A., Wheeler, T. M., Frolov, A., Scardino, P. T., Ohori, M., et al. (2003). Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 9(13), 4792–4801.
Provenzano, P. P., Cuevas, C., Chang, A. E., Goel, V. K., Von Hoff, D. D., & Hingorani, S. R. (2012). Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell, 21(3), 418–429. https://doi.org/10.1016/j.ccr.2012.01.007.
Shimosato, Y., Suzuki, A., Hashimoto, T., Nishiwaki, Y., Kodama, T., Yoneyama, T., & Kameya, T. (1980). Prognostic implications of fibrotic focus (scar) in small peripheral lung cancers. The American Journal of Surgical Pathology, 4(4), 365–373. https://doi.org/10.1097/00000478-198008000-00005.
Cardone, A., Tolino, A., Zarcone, R., Borruto Caracciolo, G., & Tartaglia, E. (1997). Prognostic value of desmoplastic reaction and lymphocytic infiltration in the management of breast cancer. Panminerva Medica, 39(3), 174–177.
Hasebe, T., Sasaki, S., Imoto, S., Mukai, K., Yokose, T., & Ochiai, A. (2002). Prognostic significance of fibrotic focus in invasive ductal carcinoma of the breast: a prospective observational study. Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc, 15(5), 502–516. https://doi.org/10.1038/modpathol.3880555.
Ueno, H., Jones, A. M., Wilkinson, K. H., Jass, J. R., & Talbot, I. C. (2004). Histological categorisation of fibrotic cancer stroma in advanced rectal cancer. Gut, 53(4), 581–586. https://doi.org/10.1136/gut.2003.028365.
Ueno, H., Konishi, T., Ishikawa, Y., Shimazaki, H., Ueno, M., Aosasa, S., et al. (2014). Histologic categorization of fibrotic cancer stroma in the primary tumor is an independent prognostic index in resectable colorectal liver metastasis. The American Journal of Surgical Pathology, 38(10), 1380–1386. https://doi.org/10.1097/PAS.0000000000000232.
Bran. (2009). Keloids: current concepts of pathogenesis (Review). International Journal of Molecular Medicine, 24(3). https://doi.org/10.3892/ijmm_00000231.
Ueno, H., Jones, A., Jass, J. R., & Talbot, I. C. (2002). Clinicopathological significance of the `keloid-like’ collagen and myxoid stroma in advanced rectal cancer. Histopathology, 40(4), 327–334. https://doi.org/10.1046/j.1365-2559.2002.01376.x.
Nearchou, I. P., Kajiwara, Y., Mochizuki, S., Harrison, D. J., Caie, P. D., & Ueno, H. (2019). Novel internationally verified method reports desmoplastic reaction as the most significant prognostic feature for disease-specific survival in stage II colorectal cancer. The American Journal of Surgical Pathology, 43(9), 1239. https://doi.org/10.1097/PAS.0000000000001304.
Shin, N., Son, G. M., Shin, D.-H., Kwon, M.-S., Park, B.-S., Kim, H.-S., et al. (2019). Cancer-associated fibroblasts and desmoplastic reactions related to cancer invasiveness in patients with colorectal cancer. Annals of Coloproctology, 35(1), 36–46. https://doi.org/10.3393/ac.2018.09.10.
Fujita, H., Ohuchida, K., Mizumoto, K., Nakata, K., Yu, J., Kayashima, T., et al. (2010). α-Smooth muscle actin expressing stroma promotes an aggressive tumor biology in pancreatic ductal adenocarcinoma. Pancreas, 39(8), 1254. https://doi.org/10.1097/MPA.0b013e3181dbf647.
Whatcott, C. J., Diep, C. H., Jiang, P., Watanabe, A., LoBello, J., Sima, C., et al. (2015). Desmoplasia in primary tumors and metastatic lesions of pancreatic cancer. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 21(15), 3561–3568. https://doi.org/10.1158/1078-0432.CCR-14-1051.
Bever, K. M., Sugar, E. A., Bigelow, E., Sharma, R., Laheru, D., Wolfgang, C. L., et al. (2015). The prognostic value of stroma in pancreatic cancer in patients receiving adjuvant therapy. HPB, 17(4), 292–298. https://doi.org/10.1111/hpb.12334.
Wang, L. M., Silva, M. A., D’Costa, Z., Bockelmann, R., Soonawalla, Z., Liu, S., et al. (2016). The prognostic role of desmoplastic stroma in pancreatic ductal adenocarcinoma. Oncotarget, 7(4), 4183–4194. https://doi.org/10.18632/oncotarget.6770.
Willumsen, N., Ali, S. M., Leitzel, K., Drabick, J. J., Yee, N., Polimera, H. V., et al. (2019). Collagen fragments quantified in serum as measures of desmoplasia associate with survival outcome in patients with advanced pancreatic cancer. Scientific Reports, 9(1), 1–8. https://doi.org/10.1038/s41598-019-56268-3.
Fang, M., Yuan, J., Peng, C., & Li, Y. (2014). Collagen as a double-edged sword in tumor progression. Tumour Biology, 35(4), 2871–2882. https://doi.org/10.1007/s13277-013-1511-7.
Perryman, L., & Erler, J. T. (2014). Lysyl oxidase in cancer research. Future Oncology (London, England), 10(9), 1709–1717. https://doi.org/10.2217/fon.14.39.
Pankova, D., Chen, Y., Terajima, M., Schliekelman, M. J., Baird, B. N., Fahrenholtz, M., et al. (2016). Cancer-associated fibroblasts induce a collagen cross-link switch in tumor stroma. Molecular Cancer Research, 14(3), 287–295. https://doi.org/10.1158/1541-7786.MCR-15-0307.
Provenzano, P. P., Inman, D. R., Eliceiri, K. W., Trier, S. M., & Keely, P. J. (2008). Contact guidance mediated three-dimensional cell migration is regulated by Rho/ROCK-dependent matrix reorganization. Biophysical Journal, 95(11), 5374–5384. https://doi.org/10.1529/biophysj.108.133116.
Zhou, Z.-H., Ji, C.-D., **ao, H.-L., Zhao, H.-B., Cui, Y.-H., & Bian, X.-W. (2017). Reorganized collagen in the tumor microenvironment of gastric cancer and its association with prognosis. Journal of Cancer, 8(8), 1466–1476. https://doi.org/10.7150/jca.18466.
Bredfeldt, J. S., Liu, Y., Conklin, M. W., Keely, P. J., Mackie, T. R., & Eliceiri, K. W. (2014). Automated quantification of aligned collagen for human breast carcinoma prognosis. Journal of Pathology Informatics, 5(1), 28. https://doi.org/10.4103/2153-3539.139707.
Franchi, M., Masola, V., Bellin, G., Onisto, M., Karamanos, K.-A., & Piperigkou, Z. (2019). Collagen fiber array of peritumoral stroma influences epithelial-to-mesenchymal transition and invasive potential of mammary cancer cells. Journal of Clinical Medicine, 8(2). https://doi.org/10.3390/jcm8020213.
Conklin, M. W., Eickhoff, J. C., Riching, K. M., Pehlke, C. A., Eliceiri, K. W., Provenzano, P. P., et al. (2011). Aligned collagen is a prognostic signature for survival in human breast carcinoma. The American Journal of Pathology, 178(3), 1221–1232. https://doi.org/10.1016/j.ajpath.2010.11.076.
Brabrand, A., Kariuki, I. I., Engstrøm, M. J., Haugen, O. A., Dyrnes, L. A., Åsvold, B. O., et al. (2015). Alterations in collagen fibre patterns in breast cancer. A premise for tumour invasiveness? APMIS, 123(1), 1–8. https://doi.org/10.1111/apm.12298.
Riching, K. M., Cox, B. L., Salick, M. R., Pehlke, C., Riching, A. S., Ponik, S. M., et al. (2014). 3D collagen alignment limits protrusions to enhance breast cancer cell persistence. Biophysical Journal, 107(11), 2546–2558. https://doi.org/10.1016/j.bpj.2014.10.035.
Acerbi, I., Cassereau, L., Dean, I., Shi, Q., Au, A., Park, C., et al. (2015). Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integrative biology : quantitative biosciences from nano to macro, 7(10), 1120–1134. https://doi.org/10.1039/c5ib00040h.
Naba, A., Clauser, K. R., Lamar, J. M., Carr, S. A., & Hynes, R. O. (2014). Extracellular matrix signatures of human mammary carcinoma identify novel metastasis promoters. eLife, 3, e01308. https://doi.org/10.7554/eLife.01308.
Zhang, H., Fredericks, T., **ong, G., Qi, Y., Rychahou, P. G., Li, J.-D., et al. (2018). Membrane associated collagen XIII promotes cancer metastasis and enhances anoikis resistance. Breast Cancer Research, 20(1), 116. https://doi.org/10.1186/s13058-018-1030-y.
**ong, G., Deng, L., Zhu, J., Rychahou, P. G., & Xu, R. (2014). Prolyl-4-hydroxylase α subunit 2 promotes breast cancer progression and metastasis by regulating collagen deposition. BMC Cancer, 14, 1. https://doi.org/10.1186/1471-2407-14-1.
Damaghi, M., Byrne, S., Xu, L., Tafreshi, N., Fang, B., Koomen, J. M., … Gillies, R. J. (2019). Collagen production and niche engineering: a novel strategy for cancer cells to survive acidosis and evolve. bioRxiv, 711978. https://doi.org/10.1101/711978
Pankova, D., Jiang, Y., Chatzifrangkeskou, M., Vendrell, I., Buzzelli, J., Ryan, A., … O’Neill, E. (2019). RASSF1A controls tissue stiffness and cancer stem-like cells in lung adenocarcinoma. The EMBO Journal, 38(13). 10.15252/embj.2018100532
Fang, S., Dai, Y., Mei, Y., Yang, M., Hu, L., Yang, H., et al. (2019). Clinical significance and biological role of cancer-derived type I collagen in lung and esophageal cancers. Thoracic Cancer, 10(2), 277–288. https://doi.org/10.1111/1759-7714.12947.
Naba, A., Clauser, K. R., Hoersch, S., Liu, H., Carr, S. A., & Hynes, R. O. (2012). The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Molecular & Cellular Proteomics : MCP, 11(4). https://doi.org/10.1074/mcp.M111.014647.
Ohlund, D., Lundin, C., Ardnor, B., Oman, M., Naredi, P., & Sund, M. (2009). Type IV collagen is a tumour stroma-derived biomarker for pancreas cancer. British Journal of Cancer, 101(1), 91–97. https://doi.org/10.1038/sj.bjc.6605107.
Ting, D. T., Wittner, B. S., Ligorio, M., Jordan, N. V., Shah, A. M., Miyamoto, D. T., et al. (2014). Single-cell RNA sequencing identifies extracellular matrix gene expression by pancreatic circulating tumor cells. Cell Reports, 8(6), 1905–1918. https://doi.org/10.1016/j.celrep.2014.08.029.
Miyake, M., Hori, S., Morizawa, Y., Tatsumi, Y., Toritsuka, M., Ohnishi, S., et al. (2017). Collagen type IV alpha 1 (COL4A1) and collagen type XIII alpha 1 (COL13A1) produced in cancer cells promote tumor budding at the invasion front in human urothelial carcinoma of the bladder. Oncotarget, 8(22), 36099–36114. https://doi.org/10.18632/oncotarget.16432.
Cavaco, A., Rezaei, M., Niland, S., & Eble, J. A. (2017). Collateral damage intended—cancer-associated fibroblasts and vasculature are potential targets in cancer therapy. International Journal of Molecular Sciences, 18(11), 2355. https://doi.org/10.3390/ijms18112355.
Liu, T., Zhou, L., Li, D., Andl, T., & Zhang, Y. (2019). Cancer-associated fibroblasts build and secure the tumor microenvironment. Frontiers in Cell and Developmental Biology, 7. https://doi.org/10.3389/fcell.2019.00060
Hanley, C. J., Noble, F., Ward, M., Bullock, M., Drifka, C., Mellone, M., et al. (2015). A subset of myofibroblastic cancer-associated fibroblasts regulate collagen fiber elongation, which is prognostic in multiple cancers. Oncotarget, 7(5), 6159–6174. https://doi.org/10.18632/oncotarget.6740.
Faouzi, S., Le Bail, B., Neaud, V., Boussarie, L., Saric, J., Bioulac-Sage, P., et al. (1999). Myofibroblasts are responsible for collagen synthesis in the stroma of human hepatocellular carcinoma: an in vivo and in vitro study. Journal of Hepatology, 30(2), 275–284. https://doi.org/10.1016/s0168-8278(99)80074-9.
Kauppila, S., Stenbäck, F., Risteli, J., Jukkola, A., & Risteli, L. (1998). Aberrant type I and type III collagen gene expression in human breast cancer in vivo. The Journal of Pathology, 186(3), 262–268. https://doi.org/10.1002/(SICI)1096-9896(1998110)186:3<262::AID-PATH191>3.0.CO;2-3.
Bauer, M., Su, G., Casper, C., He, R., Rehrauer, W., & Friedl, A. (2010). Heterogeneity of gene expression in stromal fibroblasts of human breast carcinomas and normal breast. Oncogene, 29(12), 1732–1740. https://doi.org/10.1038/onc.2009.463.
Bachem, M. G., Schneider, E., Groß, H., Weidenbach, H., Schmid, R. M., Menke, A., et al. (1998). Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology, 115(2), 421–432. https://doi.org/10.1016/S0016-5085(98)70209-4.
Kwa, M. Q., Herum, K. M., & Brakebusch, C. (2019). Cancer-associated fibroblasts: how do they contribute to metastasis? Clinical & Experimental Metastasis, 36(2), 71–86. https://doi.org/10.1007/s10585-019-09959-0.
Lambrechts, D., Wauters, E., Boeckx, B., Aibar, S., Nittner, D., Burton, O., et al. (2018). Phenotype molding of stromal cells in the lung tumor microenvironment. Nature Medicine, 24(8), 1277–1289. https://doi.org/10.1038/s41591-018-0096-5.
Lai, S. L., Tan, M. L., Hollows, R. J., Robinson, M., Ibrahim, M., Margielewska, S., et al. (2019). Collagen induces a more proliferative, migratory and chemoresistant phenotype in head and neck cancer via DDR1. Cancers, 11(11). https://doi.org/10.3390/cancers11111766.
Karagiannis, G. S., Petraki, C., Prassas, I., Saraon, P., Musrap, N., Dimitromanolakis, A., & Diamandis, E. P. (2012). Proteomic signatures of the desmoplastic invasion front reveal collagen type XII as a marker of myofibroblastic differentiation during colorectal cancer metastasis. Oncotarget, 3(3), 267–285.
Väisänen, T., Väisänen, M.-R., Autio-Harmainen, H., & Pihlajaniemi, T. (2005). Type XIII collagen expression is induced during malignant transformation in various epithelial and mesenchymal tumours. The Journal of Pathology, 207(3), 324–335. https://doi.org/10.1002/path.1836.
Karousou, E., D’Angelo, M. L., Kouvidi, K., Vigetti, D., Viola, M., Nikitovic, D., … Passi, A. (2014). Collagen VI and hyaluronan: the common role in breast cancer. BioMed Research International. Research article. https://doi.org/10.1155/2014/606458
Schnoor, M., Cullen, P., Lorkowski, J., Stolle, K., Robenek, H., Troyer, D., et al. (2008). Production of type VI collagen by human macrophages: a new dimension in macrophage functional heterogeneity. Journal of Immunology (Baltimore, Md.: 1950), 180(8), 5707–5719. https://doi.org/10.4049/jimmunol.180.8.5707.
Varol, C., & Sagi, I. (2018). Phagocyte—extracellular matrix crosstalk empowers tumor development and dissemination. The FEBS Journal, 285(4), 734–751. https://doi.org/10.1111/febs.14317.
Afik, R., Zigmond, E., Vugman, M., Klepfish, M., Shimshoni, E., Pasmanik-Chor, M., et al. (2016). Tumor macrophages are pivotal constructors of tumor collagenous matrix. The Journal of Experimental Medicine, 213(11), 2315–2331. https://doi.org/10.1084/jem.20151193.
Ingman, W. V., Wyckoff, J., Gouon-Evans, V., Condeelis, J., & Pollard, J. W. (2006). Macrophages promote collagen fibrillogenesis around terminal end buds of the develo** mammary gland. Developmental Dynamics: An Official Publication of the American Association of the Anatomists, 235(12), 3222–3229. https://doi.org/10.1002/dvdy.20972.
Casimiro, S., Ferreira, A. R., Mansinho, A., Alho, I., & Costa, L. (2016). Molecular mechanisms of bone metastasis: which targets came from the bench to the bedside? International Journal of Molecular Sciences, 17(9). https://doi.org/10.3390/ijms17091415.
Condeelis, J., & Segall, J. E. (2003). Intravital imaging of cell movement in tumours. Nature Reviews Cancer, 3(12), 921–930. https://doi.org/10.1038/nrc1231.
Qiu, S., Deng, L., Liao, X., Nie, L., Qi, F., **, K., et al. (2019). Tumor-associated macrophages promote bladder tumor growth through PI3K/AKT signal induced by collagen. Cancer Science, 110(7), 2110–2118. https://doi.org/10.1111/cas.14078.
Wei, X., Li, S., He, J., Du, H., Liu, Y., Yu, W., et al. (2019). Tumor-secreted PAI-1 promotes breast cancer metastasis via the induction of adipocyte-derived collagen remodeling. Cell Communication and Signaling: CCS, 17(1), 58. https://doi.org/10.1186/s12964-019-0373-z.
Iyengar, P., Espina, V., Williams, T. W., Lin, Y., Berry, D., Jelicks, L. A., et al. (2005). Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. Journal of Clinical Investigation, 115(5), 1163–1176. https://doi.org/10.1172/JCI200523424.
Weilbaecher, K. N., Guise, T. A., & McCauley, L. K. (2011). Cancer to bone: a fatal attraction. Nature Reviews. Cancer, 11(6), 411–425. https://doi.org/10.1038/nrc3055
Kolb, A. D., & Bussard, K. M. (2019). The bone extracellular matrix as an ideal milieu for cancer cell metastases. Cancers, 11(7). https://doi.org/10.3390/cancers11071020.
Januchowski, R., Zawierucha, P., Ruciński, M., Nowicki, M., & Zabel, M. (2014). Extracellular matrix proteins expression profiling in chemoresistant variants of the A2780 ovarian cancer cell line. BioMed Research International, 2014, 365867. https://doi.org/10.1155/2014/365867.
Kanematsu, A., Marui, A., Yamamoto, S., Ozeki, M., Hirano, Y., Yamamoto, M., et al. (2004). Type I collagen can function as a reservoir of basic fibroblast growth factor. Journal of Controlled Release, 99(2), 281–292. https://doi.org/10.1016/j.jconrel.2004.07.008.
Jain, R. K. (2003). Molecular regulation of vessel maturation. Nature Medicine, 9(6), 685–693. https://doi.org/10.1038/nm0603-685.
Seandel, M., Noack-Kunnmann, K., Zhu, D., Aimes, R. T., & Quigley, J. P. (2001). Growth factor-induced angiogenesis in vivo requires specific cleavage of fibrillar type I collagen. Blood, 97(8), 2323–2332. https://doi.org/10.1182/blood.v97.8.2323.
Menke, A., Philippi, C., Vogelmann, R., Seidel, B., Lutz, M. P., Adler, G., & Wedlich, D. (2001). Down-regulation of E-cadherin gene expression by collagen type I and type III in pancreatic cancer cell lines. Cancer Research, 61(8), 3508–3517.
Shields, M. A., Dangi-Garimella, S., Krantz, S. B., Bentrem, D. J., & Munshi, H. G. (2011). Pancreatic cancer cells respond to type I collagen by inducing snail expression to promote membrane type 1 matrix metalloproteinase-dependent collagen invasion. The Journal of Biological Chemistry, 286(12), 10495–10504. https://doi.org/10.1074/jbc.M110.195628.
Jang, M., Koh, I., Lee, J. E., Lim, J. Y., Cheong, J.-H., & Kim, P. (2018). Increased extracellular matrix density disrupts E-cadherin/β-catenin complex in gastric cancer cells. Biomaterials Science, 6(10), 2704–2713. https://doi.org/10.1039/C8BM00843D.
Di Martino, J., Moreau, V., & Saltel, F. (2015). Type I collagen fibrils: an inducer of invadosomes. Oncotarget, 6(30), 28519–28520.
Juin, A., Billottet, C., Moreau, V., Destaing, O., Albiges-Rizo, C., Rosenbaum, J., et al. (2012). Physiological type I collagen organization induces the formation of a novel class of linear invadosomes. Molecular Biology of the Cell, 23(2), 297–309. https://doi.org/10.1091/mbc.E11-07-0594.
Juin, A., Di Martino, J., Leitinger, B., Henriet, E., Gary, A.-S., Paysan, L., et al. (2014). Discoidin domain receptor 1 controls linear invadosome formation via a Cdc42-Tuba pathway. The Journal of Cell Biology, 207(4), 517–533. https://doi.org/10.1083/jcb.201404079.
Gao, H., Chakraborty, G., Zhang, Z., Akalay, I., Gadiya, M., Gao, Y., et al. (2016). Multi-organ site metastatic reactivation mediated by non-canonical discoidin domain receptor 1 signaling. Cell, 166(1), 47–62. https://doi.org/10.1016/j.cell.2016.06.009.
Barcus, C. E., O’Leary, K. A., Brockman, J. L., Rugowski, D. E., Liu, Y., Garcia, N., et al. (2017). Elevated collagen-I augments tumor progressive signals, intravasation and metastasis of prolactin-induced estrogen receptor alpha positive mammary tumor cells. Breast cancer research: BCR, 19(1), 9. https://doi.org/10.1186/s13058-017-0801-1.
Hall, C. L., Dai, J., van Golen, K. L., Keller, E. T., & Long, M. W. (2006). Type I collagen receptor (alpha 2 beta 1) signaling promotes the growth of human prostate cancer cells within the bone. Cancer Research, 66(17), 8648–8654. https://doi.org/10.1158/0008-5472.CAN-06-1544.
Clarke, C. J., Berg, T. J., Birch, J., Ennis, D., Mitchell, L., Cloix, C., et al. (2016). The initiator methionine tRNA drives secretion of type II collagen from stromal fibroblasts to promote tumor growth and angiogenesis. Current Biology, 26(6), 755–765. https://doi.org/10.1016/j.cub.2016.01.045.
Chintala, S. K., Sawaya, R., Gokaslan, Z. L., & Rao, J. S. (1996). The effect of type III collagen on migration and invasion of human glioblastoma cell lines in vitro. Cancer Letters, 102(1–2), 57–63. https://doi.org/10.1016/0304-3835(96)04163-8.
Wang, Z.-N., & Xu, H.-M. (2000). Relationship between collagen IV expression and biological behavior of gastric cancer. World Journal of Gastroenterology, 6(3), 438–439. https://doi.org/10.3748/wjg.v6.i3.438.
Öhlund, D., Franklin, O., Lundberg, E., Lundin, C., & Sund, M. (2013). Type IV collagen stimulates pancreatic cancer cell proliferation, migration, and inhibits apoptosis through an autocrine loop. BMC Cancer, 13, 154. https://doi.org/10.1186/1471-2407-13-154.
Aznavoorian, S., Stracke, M. L., Krutzsch, H., Schiffmann, E., & Liotta, L. A. (1990). Signal transduction for chemotaxis and haptotaxis by matrix molecules in tumor cells. The Journal of Cell Biology, 110(4), 1427–1438. https://doi.org/10.1083/jcb.110.4.1427.
Barsky, S. H., Rao, C. N., Grotendorst, G. R., & Liotta, L. A. (1982). Increased content of type V collagen in desmoplasia of human breast carcinoma. The American Journal of Pathology, 108(3), 276–283.
Huang, G., Ge, G., Izzi, V., & Greenspan, D. S. (2017). α3 Chains of type V collagen regulate breast tumour growth via glypican-1. Nature Communications, 8. https://doi.org/10.1038/ncomms14351.
Berchtold, S., Grünwald, B., Krüger, A., Reithmeier, A., Hähl, T., Cheng, T., et al. (2015). Collagen type V promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Cancer Letters, 356(2 Pt B), 721–732. https://doi.org/10.1016/j.canlet.2014.10.020.
Wright, A., Li, Y.-H., & Zhu, C. (2008). The differential effect of endothelial cell factors on in vitro motility of malignant and non-malignant cells. Annals of Biomedical Engineering, 36(6), 958–969. https://doi.org/10.1007/s10439-008-9489-9.
Huang, Y., Li, G., Wang, K., Mu, Z., **e, Q., Qu, H., et al. (2018). Collagen type VI alpha 3 chain promotes epithelial-mesenchymal transition in bladder cancer cells via transforming growth factor β (TGF-β)/Smad pathway. Medical Science Monitor : International Medical Journal of Experimental and Clinical Research, 24, 5346–5354. https://doi.org/10.12659/MSM.909811.
Owusu-Ansah, K. G., Song, G., Chen, R., Edoo, M. I. A., Li, J., Chen, B., et al. (2019). COL6A1 promotes metastasis and predicts poor prognosis in patients with pancreatic cancer. International Journal of Oncology, 55(2), 391–404. https://doi.org/10.3892/ijo.2019.4825.
You, W.-K., Bonaldo, P., & Stallcup, W. B. (2012). Collagen VI ablation retards brain tumor progression due to deficits in assembly of the vascular basal lamina. The American Journal of Pathology, 180(3), 1145–1158. https://doi.org/10.1016/j.ajpath.2011.11.006.
Martins, V. L., Caley, M. P., Moore, K., Szentpetery, Z., Marsh, S. T., Murrell, D. F., et al. (2016). Suppression of TGFβ and angiogenesis by type VII collagen in cutaneous SCC. Journal of the National Cancer Institute, 108(1). https://doi.org/10.1093/jnci/djv293.
Oktem, G., Sercan, O., Guven, U., Uslu, R., Uysal, A., Goksel, G., et al. (2014). Cancer stem cell differentiation: TGFβ1 and versican may trigger molecules for the organization of tumor spheroids. Oncology Reports, 32(2), 641–649. https://doi.org/10.3892/or.2014.3252.
Zhao, Y., Jia, L., Mao, X., Xu, H., Wang, B., & Liu, Y. (2009). siRNA-targeted COL8A1 inhibits proliferation, reduces invasion and enhances sensitivity to D-limonence treatment in hepatocarcinoma cells. IUBMB Life, 61(1), 74–79. https://doi.org/10.1002/iub.151.
ang, W., Xu, G., Ding, C.-L., Zhao, L.-J., Zhao, P., Ren, H., & Qi, Z.-T. (2013). All-trans retinoic acid protects hepatocellular carcinoma cells against serum-starvation-induced cell death by upregulating collagen 8A2. The FEBS Journal, 280(5), 1308–1319. https://doi.org/10.1111/febs.12122.
Chapman, K. B., Prendes, M. J., Sternberg, H., Kidd, J. L., Funk, W. D., Wagner, J., & West, M. D. (2012). COL10A1 expression is elevated in diverse solid tumor types and is associated with tumor vasculature. Future Oncology (London, England), 8(8), 1031–1040. https://doi.org/10.2217/fon.12.79.
Huang, H., Li, T., Ye, G., Zhao, L., Zhang, Z., Mo, D., et al. (2018). High expression of COL10A1 is associated with poor prognosis in colorectal cancer. OncoTargets and Therapy, 11, 1571–1581. https://doi.org/10.2147/OTT.S160196.
van Huizen, N. A., Coebergh van den Braak, R. R. J., Doukas, M., Dekker, L. J. M., IJzermans, J. N. M., & Luider, T. M. (2019). Up-regulation of collagen proteins in colorectal liver metastasis compared with normal liver tissue. The Journal of Biological Chemistry, 294(1), 281–289. https://doi.org/10.1074/jbc.RA118.005087.
Fischer, H., Stenling, R., Rubio, C., & Lindblom, A. (2001). Colorectal carcinogenesis is associated with stromal expression of COL11A1 and COL5A2. Carcinogenesis, 22(6), 875–878. https://doi.org/10.1093/carcin/22.6.875.
Shen, L., Yang, M., Lin, Q., Zhang, Z., Zhu, B., & Miao, C. (2016). COL11A1 is overexpressed in recurrent non-small cell lung cancer and promotes cell proliferation, migration, invasion and drug resistance. Oncology Reports, 36(2), 877–885. https://doi.org/10.3892/or.2016.4869.
Zhao, Y., Zhou, T., Li, A., Yao, H., He, F., Wang, L., & Si, J. (2009). A potential role of collagens expression in distinguishing between premalignant and malignant lesions in stomach. Anatomical Record (Hoboken, N.J.: 2007), 292(5), 692–700. https://doi.org/10.1002/ar.20874.
García-Pravia, C., Galván, J. A., Gutiérrez-Corral, N., Solar-García, L., García-Pérez, E., García-Ocaña, M., et al. (2013). Overexpression of COL11A1 by cancer-associated fibroblasts: clinical relevance of a stromal marker in pancreatic cancer. PLoS One, 8(10), e78327. https://doi.org/10.1371/journal.pone.0078327.
Feng, Y., Sun, B., Li, X., Zhang, L., Niu, Y., **ao, C., et al. (2007). Differentially expressed genes between primary cancer and paired lymph node metastases predict clinical outcome of node-positive breast cancer patients. Breast Cancer Research and Treatment, 103(3), 319–329. https://doi.org/10.1007/s10549-006-9385-7.
Sok, J. C., Lee, J. A., Dasari, S., Joyce, S., Contrucci, S. C., Egloff, A. M., et al. (2013). Collagen type XI α1 facilitates head and neck squamous cell cancer growth and invasion. British Journal of Cancer, 109(12), 3049–3056. https://doi.org/10.1038/bjc.2013.624.
Yen, T.-Y., Haste, N., Timpe, L. C., Litsakos-Cheung, C., Yen, R., & Macher, B. A. (2014). Using a cell line breast cancer progression system to identify biomarker candidates. Journal of Proteomics, 96, 173–183. https://doi.org/10.1016/j.jprot.2013.11.006.
Reddy, L. A., Mikesh, L., Moskulak, C., Harvey, J., Sherman, N., Zigrino, P., et al. (2014). Host response to human breast invasive ductal carcinoma (IDC) as observed by changes in the stromal proteome. Journal of Proteome Research, 13(11), 4739–4751. https://doi.org/10.1021/pr500620x.
Verghese, E. T., Drury, R., Green, C. A., Holliday, D. L., Lu, X., Nash, C., et al. (2013). MiR-26b is down-regulated in carcinoma-associated fibroblasts from ER-positive breast cancers leading to enhanced cell migration and invasion. The Journal of Pathology, 231(3), 388–399. https://doi.org/10.1002/path.4248.
Karagiannis, G. S., Petraki, C., Prassas, I., Saraon, P., Musrap, N., Dimitromanolakis, A., & Diamandis, E. P. (2012). Proteomic signatures of the desmoplastic invasion front reveal collagen type XII as a marker of myofibroblastic differentiation during colorectal cancer metastasis. Oncotarget, 3(3), 267–285. https://doi.org/10.18632/oncotarget.451.
Hurskainen, M., Ruggiero, F., Hägg, P., Pihlajaniemi, T., & Huhtala, P. (2010). Recombinant human collagen XV regulates cell adhesion and migration. The Journal of Biological Chemistry, 285(8), 5258–5265. https://doi.org/10.1074/jbc.M109.033787.
Clementz, A. G., & Harris, A. (2013). Collagen XV: exploring its structure and its role within the tumor microenvironment. Molecular cancer research : MCR, 11(12), 1481–1486. https://doi.org/10.1158/1541-7786.MCR-12-0662.
Bauer, R., Ratzinger, S., Wales, L., Bosserhoff, A., Senner, V., Grifka, J., & Grässel, S. (2011). Inhibition of collagen XVI expression reduces glioma cell invasiveness. Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology, 27(3–4), 217–226. https://doi.org/10.1159/000327947.
Ratzinger, S., Grässel, S., Dowejko, A., Reichert, T. E., & Bauer, R. J. (2011). Induction of type XVI collagen expression facilitates proliferation of oral cancer cells. Matrix Biology: Journal of the International Society for Matrix Biology, 30(2), 118–125. https://doi.org/10.1016/j.matbio.2011.01.001.
Maegdefrau, U., & Bosserhoff, A.-K. (2012). BMP activated Smad signaling strongly promotes migration and invasion of hepatocellular carcinoma cells. Experimental and Molecular Pathology, 92(1), 74–81. https://doi.org/10.1016/j.yexmp.2011.10.004.
Banyard, J., Bao, L., Hofer, M. D., Zurakowski, D., Spivey, K. A., Feldman, A. S., et al. (2007). Collagen XXIII expression is associated with prostate cancer recurrence and distant metastases. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 13(9), 2634–2642. https://doi.org/10.1158/1078-0432.CCR-06-2163.
Spivey, K. A., Chung, I., Banyard, J., Adini, I., Feldman, H. A., & Zetter, B. R. (2012). A role for collagen XXIII in cancer cell adhesion, anchorage-independence, and metastasis. Oncogene, 31(18), 2362–2372. https://doi.org/10.1038/onc.2011.406.
Najafi, M., Farhood, B., & Mortezaee, K. (2019). Extracellular matrix (ECM) stiffness and degradation as cancer drivers. Journal of Cellular Biochemistry, 120(3), 2782–2790. https://doi.org/10.1002/jcb.27681.
Monboisse, J. C., Oudart, J. B., Ramont, L., Brassart-Pasco, S., & Maquart, F. X. (2014). Matrikines from basement membrane collagens: a new anti-cancer strategy. Biochimica et Biophysica Acta, 1840(8), 2589–2598. https://doi.org/10.1016/j.bbagen.2013.12.029.
Palmieri, D., Camardella, L., Ulivi, V., Guasco, G., & Manduca, P. (2000). Trimer carboxyl propeptide of collagen I produced by mature osteoblasts is chemotactic for endothelial cells. The Journal of Biological Chemistry, 275(42), 32658–32663. https://doi.org/10.1074/jbc.M002698200.
Palmieri, D., Astigiano, S., Barbieri, O., Ferrari, N., Marchisio, S., Ulivi, V., et al. (2008). Procollagen I COOH-terminal fragment induces VEGF-A and CXCR4 expression in breast carcinoma cells. Experimental Cell Research, 314(11), 2289–2298. https://doi.org/10.1016/j.yexcr.2008.04.016.
Visigalli, D., Palmieri, D., Strangio, A., Astigiano, S., Barbieri, O., Casartelli, G., et al. (2009). The carboxyl terminal trimer of procollagen I induces pro-metastatic changes and vascularization in breast cancer cells xenografts. BMC Cancer, 9, 59. https://doi.org/10.1186/1471-2407-9-59.
Hayashi, S., Wang, Z., Bryan, J., Kobayashi, C., Faccio, R., & Sandell, L. J. (2011). The type II collagen N-propeptide, PIIBNP, inhibits cell survival and bone resorption of osteoclasts via integrin-mediated signaling. Bone, 49(4), 644–652. https://doi.org/10.1016/j.bone.2011.06.011.
Wang, Z., Bryan, J., Franz, C., Havlioglu, N., & Sandell, L. J. (2010). Type IIB procollagen NH(2)-propeptide induces death of tumor cells via interaction with integrins alpha(V)beta(3) and alpha(V)beta(5). The Journal of Biological Chemistry, 285(27), 20806–20817. https://doi.org/10.1074/jbc.M110.118521.
Sandell, L. J., Wang, Z., Franz, C., Bryan, J., Siegel, A., Mecham, R., et al. (2008). Live-cell imaging of endothelial cell tube formation: inhibition by chondrostatin. The FASEB Journal, 22(1_supplement), 101.4–101.4. https://doi.org/10.1096/fasebj.22.1_supplement.101.4.
Wang, Z., Bryan, J., Franz, C., Siegel, A., Wagenseil, J., Mecham, R., & Sandell, L. J. (2008). A fragment of cartilage collagen, chondrostatin, inhibits migration of breast cancer cells. The FASEB Journal, 22(1_supplement), 1029.11–1029.11. https://doi.org/10.1096/fasebj.22.1_supplement.1029.11.
Ghajar, C. M., George, S. C., & Putnam, A. J. (2008). Matrix metalloproteinase control of capillary morphogenesis. Critical Reviews in Eukaryotic Gene Expression, 18(3), 251–278.
Lv, Y., & Zheng, J. (2012). The inhibitory effects of Arresten protein on tumor formation. Chinese Medical Sciences Journal, 27(1), 11–17. https://doi.org/10.1016/S1001-9294(12)60016-9.
Aikio, M., Alahuhta, I., Nurmenniemi, S., Suojanen, J., Palovuori, R., Teppo, S., et al. (2012). Arresten, a collagen-derived angiogenesis inhibitor, suppresses invasion of squamous cell carcinoma. PLoS One, 7(12). https://doi.org/10.1371/journal.pone.0051044.
Okada, M., & Yamawaki, H. (2019). A current perspective of canstatin, a fragment of type IV collagen alpha 2 chain. Journal of Pharmacological Sciences, 139(2), 59–64. https://doi.org/10.1016/j.jphs.2018.12.001.
Kamphaus, G. D., Colorado, P. C., Panka, D. J., Hopfer, H., Ramchandran, R., Torre, A., et al. (2000). Canstatin, a novel matrix-derived inhibitor of angiogenesis and tumor growth. The Journal of Biological Chemistry, 275(2), 1209–1215. https://doi.org/10.1074/jbc.275.2.1209.
He, G.-A., Luo, J.-X., Zhang, T.-Y., Wang, F.-Y., & Li, R.-F. (2003). Canstatin-N fragment inhibits in vitro endothelial cell proliferation and suppresses in vivo tumor growth. Biochemical and Biophysical Research Communications, 312(3), 801–805. https://doi.org/10.1016/j.bbrc.2003.11.003.
Maeshima, Y., Manfredi, M., Reimer, C., Holthaus, K. A., Hopfer, H., Chandamuri, B. R., et al. (2001). Identification of the anti-angiogenic site within vascular basement membrane-derived tumstatin. The Journal of Biological Chemistry, 276(18), 15240–15248. https://doi.org/10.1074/jbc.M007764200.
Sudhakar, A., Sugimoto, H., Yang, C., Lively, J., Zeisberg, M., & Kalluri, R. (2003). Human tumstatin and human endostatin exhibit distinct antiangiogenic activities mediated by alpha v beta 3 and alpha 5 beta 1 integrins. Proceedings of the National Academy of Sciences of the United States of America, 100(8), 4766–4771. https://doi.org/10.1073/pnas.0730882100.
Mott, J. D., & Werb, Z. (2004). Regulation of matrix biology by matrix metalloproteinases. Current Opinion in Cell Biology, 16(5), 558–564. https://doi.org/10.1016/j.ceb.2004.07.010.
Brassart-Pasco, S., Sénéchal, K., Thevenard, J., Ramont, L., Devy, J., Stefano, L. D., et al. (2012). Tetrastatin, the NC1 domain of the α4(IV) collagen chain: a novel potent anti-tumor matrikine. PLoS One, 7(4), e29587. https://doi.org/10.1371/journal.pone.0029587.
Lambert, E., Fuselier, E., Ramont, L., Brassart, B., Dukic, S., Oudart, J.-B., et al. (2018). Conformation-dependent binding of a Tetrastatin peptide to α v β 3 integrin decreases melanoma progression through FAK/PI 3 K/Akt pathway inhibition. Scientific Reports, 8(1), 1–13. https://doi.org/10.1038/s41598-018-28003-x.
Karagiannis, E. D., & Popel, A. S. (2007). Identification of novel short peptides derived from the α4, α5, and α6 fibrils of type IV collagen with anti-angiogenic properties. Biochemical and Biophysical Research Communications, 354(2), 434–439. https://doi.org/10.1016/j.bbrc.2006.12.231.
Koskimaki, J. E., Karagiannis, E. D., Tang, B. C., Hammers, H., Watkins, D. N., Pili, R., & Popel, A. S. (2010). Pentastatin-1, a collagen IV derived 20-mer peptide, suppresses tumor growth in a small cell lung cancer xenograft model. BMC Cancer, 10(1), 29. https://doi.org/10.1186/1471-2407-10-29.
Motrescu, E. R., Blaise, S., Etique, N., Messaddeq, N., Chenard, M.-P., Stoll, I., et al. (2008). Matrix metalloproteinase-11/stromelysin-3 exhibits collagenolytic function against collagen VI under normal and malignant conditions. Oncogene, 27(49), 6347–6355. https://doi.org/10.1038/onc.2008.218.
Park, J., & Scherer, P. E. (2012). Adipocyte-derived endotrophin promotes malignant tumor progression. The Journal of Clinical Investigation, 122(11), 4243–4256. https://doi.org/10.1172/JCI63930.
Ortiz-Urda, S., Garcia, J., Green, C. L., Chen, L., Lin, Q., Veitch, D. P., et al. (2005). Type VII collagen is required for Ras-driven human epidermal tumorigenesis. Science (New York, N.Y.), 307(5716), 1773–1776. https://doi.org/10.1126/science.1106209.
Xu, R., Yao, Z.-Y., **n, L., Zhang, Q., Li, T.-P., & Gan, R.-B. (2001). NC1 domain of human type VIII collagen (α 1) inhibits bovine aortic endothelial cell proliferation and causes cell apoptosis. Biochemical and Biophysical Research Communications, 289(1), 264–268. https://doi.org/10.1006/bbrc.2001.5970.
Shen, Z., Yao, C., Wang, Z., Yue, L., Fang, Z., Yao, H., et al. (2016). Vastatin, an endogenous antiangiogenesis polypeptide that is lost in hepatocellular carcinoma, effectively inhibits tumor metastasis. Molecular Therapy, 24(8), 1358–1368. https://doi.org/10.1038/mt.2016.56.
Li, Y., Li, J., Woo, Y. M., Shen, Z., Yao, H., Cai, Y., et al. (2017). Enhanced expression of Vastatin inhibits angiogenesis and prolongs survival in murine orthotopic glioblastoma model. BMC Cancer, 17(1), 126. https://doi.org/10.1186/s12885-017-3125-8.
Willumsen, N., Jorgensen, L. N., & Karsdal, M. A. (2019). Vastatin (the NC1 domain of human type VIII collagen a1 chain) is linked to stromal reactivity and elevated in serum from patients with colorectal cancer. Cancer Biology & Therapy, 20(5), 692–699. https://doi.org/10.1080/15384047.2018.1550571.
Ramchandran, R., Dhanabal, M., Volk, R., Waterman, M. J. F., Segal, M., Lu, H., et al. (1999). Antiangiogenic activity of Restin, NC10 domain of human collagen XV: comparison to endostatin. Biochemical and Biophysical Research Communications, 255(3), 735–739. https://doi.org/10.1006/bbrc.1999.0248.
Mutolo, M. J., Morris, K. J., Leir, S.-H., Caffrey, T. C., Lewandowska, M. A., Hollingsworth, M. A., & Harris, A. (2012). Tumor suppression by collagen XV is independent of the restin domain. Matrix Biology, 31(5), 285–289. https://doi.org/10.1016/j.matbio.2012.03.003.
O’Reilly, M. S., Boehm, T., Shing, Y., Fukai, N., Vasios, G., Lane, W. S., et al. (1997). Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell, 88(2), 277–285. https://doi.org/10.1016/S0092-8674(00)81848-6.
Boehm, T., Folkman, J., Browder, T., & O’Reilly, M. S. (1997). Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature, 390(6658), 404–407. https://doi.org/10.1038/37126.
Felbor, U., Dreier, L., Bryant, R. A. R., Ploegh, H. L., Olsen, B. R., & Mothes, W. (2000). Secreted cathepsin L generates endostatin from collagen XVIII. The EMBO Journal, 19(6), 1187–1194. https://doi.org/10.1093/emboj/19.6.1187.
Kim, Y.-M., Hwang, S., Kim, Y.-M., Pyun, B.-J., Kim, T.-Y., Lee, S.-T., et al. (2002). Endostatin blocks vascular endothelial growth factor-mediated signaling via direct interaction with KDR/Flk-1. The Journal of Biological Chemistry, 277(31), 27872–27879. https://doi.org/10.1074/jbc.M202771200.
Hanai, J., Dhanabal, M., Karumanchi, S. A., Albanese, C., Waterman, M., Chan, B., et al. (2002). Endostatin causes G1 arrest of endothelial cells through inhibition of cyclin D1. Journal of Biological Chemistry, 277(19), 16464–16469. https://doi.org/10.1074/jbc.M112274200.
Bagley, R. G. (2010). The tumor microenvironment. Springer Science & Business Media.
Raglow, Z., & Thomas, S. M. (2015). Tumor matrix protein collagen XIα1 in cancer. Cancer Letters, 357(2), 448–453. https://doi.org/10.1016/j.canlet.2014.12.011.
Vázquez-Villa, F., García-Ocaña, M., Galván, J. A., García-Martínez, J., García-Pravia, C., Menéndez-Rodríguez, P., et al. (2015). COL11A1/(pro)collagen 11A1 expression is a remarkable biomarker of human invasive carcinoma-associated stromal cells and carcinoma progression. Tumour Biology: The Journal of the International Society for Oncodevelopmental Biology and Medicine, 36(4), 2213–2222. https://doi.org/10.1007/s13277-015-3295-4.
Kim, H., Watkinson, J., Varadan, V., & Anastassiou, D. (2010). Multi-cancer computational analysis reveals invasion-associated variant of desmoplastic reaction involving INHBA, THBS2 and COL11A1. BMC Medical Genomics, 3(1), 51. https://doi.org/10.1186/1755-8794-3-51.
Chong, I.-W., Chang, M.-Y., Chang, H.-C., Yu, Y.-P., Sheu, C.-C., Tsai, J.-R., et al. (2006). Great potential of a panel of multiple hMTH1, SPD, ITGA11 and COL11A1 markers for diagnosis of patients with non-small cell lung cancer. Oncology Reports, 16(5), 981–988.
Ewald, J. A., Downs, T. M., Cetnar, J. P., & Ricke, W. A. (2013). Expression microarray meta-analysis identifies genes associated with Ras/MAPK and related pathways in progression of muscle-invasive bladder transition cell carcinoma. PLoS One, 8(2), e55414. https://doi.org/10.1371/journal.pone.0055414.
Cheon, D.-J., Tong, Y., Sim, M.-S., Dering, J., Berel, D., Cui, X., et al. (2014). A collagen-remodeling gene signature regulated by TGF-β signaling is associated with metastasis and poor survival in serous ovarian cancer. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 20(3), 711–723. https://doi.org/10.1158/1078-0432.CCR-13-1256.
Suceveanu, A. I., Suceveanu, A., Voinea, F., Mazilu, L., Mixici, F., & Adam, T. (2009). Introduction of cytogenetic tests in colorectal cancer screening. Journal of gastrointestinal and liver diseases: JGLD, 18(1), 33–38.
Fischer, H., Salahshor, S., Stenling, R., Björk, J., Lindmark, G., Iselius, L., et al. (2001). COL11A1 in FAP polyps and in sporadic colorectal tumors. BMC Cancer, 1, 17. https://doi.org/10.1186/1471-2407-1-17.
Iizasa, T., Chang, H., Suzuki, M., Otsuji, M., Yokoi, S., Chiyo, M., et al. (2004). Overexpression of collagen XVIII is associated with poor outcome and elevated levels of circulating serum endostatin in non-small cell lung cancer. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 10(16), 5361–5366. https://doi.org/10.1158/1078-0432.CCR-04-0443.
Hu, T.-H., Huang, C.-C., Wu, C.-L., Lin, P.-R., Liu, S.-Y., Lin, J.-W., et al. (2005). Increased endostatin/collagen XVIII expression correlates with elevated VEGF level and poor prognosis in hepatocellular carcinoma. Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc, 18(5), 663–672. https://doi.org/10.1038/modpathol.3800336.
Thangavelu, P. U., Krenács, T., Dray, E., & Duijf, P. H. G. (2016). In epithelial cancers, aberrant COL17A1 promoter methylation predicts its misexpression and increased invasion. Clinical Epigenetics, 8(1), 120. https://doi.org/10.1186/s13148-016-0290-6.
Parikka, M., Kainulainen, T., Tasanen, K., Väänänen, A., Bruckner-Tuderman, L., & Salo, T. (2003). Alterations of collagen XVII expression during transformation of oral epithelium to dysplasia and carcinoma. The Journal of Histochemistry and Cytochemistry: Official Journal of the Histochemistry Society, 51(7), 921–929. https://doi.org/10.1177/002215540305100707.
Chen, I. M., Willumsen, N., Dehlendorff, C., Johansen, A. Z., Jensen, B. V., Hansen, C. P., et al. (2019). Clinical value of serum hyaluronan and propeptide of type III collagen in patients with pancreatic cancer. International Journal of Cancer. https://doi.org/10.1002/ijc.32751.
Banys-Paluchowski, M., Loibl, S., Witzel, I., Mundhenke, C., Lederer, B., Solbach, C., et al. (2019). Clinical relevance of collagen protein degradation markers C3M and C4M in the serum of breast cancer patients treated with neoadjuvant therapy in the GeparQuinto trial. Cancers, 11(8). https://doi.org/10.3390/cancers11081186.
Lipton, A., Leitzel, K., Ali, S. M., Polimera, H. V., Nagabhairu, V., Marks, E., et al. (2018). High turnover of extracellular matrix reflected by specific protein fragments measured in serum is associated with poor outcomes in two metastatic breast cancer cohorts. International Journal of Cancer, 143(11), 3027–3034. https://doi.org/10.1002/ijc.31627.
Kehlet, S. N., Sanz-Pamplona, R., Brix, S., Leeming, D. J., Karsdal, M. A., & Moreno, V. (2016). Excessive collagen turnover products are released during colorectal cancer progression and elevated in serum from metastatic colorectal cancer patients. Scientific Reports, 6(1), 1–7. https://doi.org/10.1038/srep30599.
Ali, S. M., Demers, L. M., Leitzel, K., Harvey, H. A., Clemens, D., Mallinak, N., et al. (2004). Baseline serum NTx levels are prognostic in metastatic breast cancer patients with bone-only metastasis. Annals of Oncology: Official Journal of the European Society for Medical Oncology, 15(3), 455–459. https://doi.org/10.1093/annonc/mdh089.
Ylisirniö, S., Höyhtyä, M., Mäkitaro, R., Pääakkö, P., Risteli, J., Kinnula, V. L., et al. (2001). Elevated serum levels of type I collagen degradation marker ICTP and tissue inhibitor of metalloproteinase (TIMP) 1 are associated with poor prognosis in lung cancer. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 7(6), 1633–1637.
Akimoto, S., Inomiya, H., Furuya, Y., Akakura, K., & Ito, H. (1998). Prognostic value of the serum levels of bone formation and bone resorption markers in prostate cancer patients with bone metastasis. European Urology, 34(2), 142–147. https://doi.org/10.1159/000019700.
Xu, S., Xu, H., Wang, W., Li, S., Li, H., Li, T., et al. (2019). The role of collagen in cancer: from bench to bedside. Journal of Translational Medicine, 17(1), 309. https://doi.org/10.1186/s12967-019-2058-1.
Zhong, S., Jeong, J.-H., Chen, Z., Chen, Z., & Luo, J.-L. (2020). Targeting tumor microenvironment by small-molecule inhibitors. Translational Oncology, 13(1), 57–69. https://doi.org/10.1016/j.tranon.2019.10.001.
Li, M., Li, M., Yin, T., Shi, H., Wen, Y., Zhang, B., et al. (2016). Targeting of cancer-associated fibroblasts enhances the efficacy of cancer chemotherapy by regulating the tumor microenvironment. Molecular Medicine Reports, 13(3), 2476–2484. https://doi.org/10.3892/mmr.2016.4868.
Mertens, J. C., Fingas, C. D., Christensen, J. D., Smoot, R. L., Bronk, S. F., Werneburg, N. W., et al. (2013). Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma. Cancer Research, 73(2), 897–907. https://doi.org/10.1158/0008-5472.CAN-12-2130.
Cleary, J. M., Lima, C. M. S. R., Hurwitz, H. I., Montero, A. J., Franklin, C., Yang, J., et al. (2014). A phase I clinical trial of navitoclax, a targeted high-affinity Bcl-2 family inhibitor, in combination with gemcitabine in patients with solid tumors. Investigational New Drugs, 32(5), 937–945. https://doi.org/10.1007/s10637-014-0110-9.
Karasic, T. B., O’Hara, M. H., Loaiza-Bonilla, A., Reiss, K. A., Teitelbaum, U. R., Borazanci, E., et al. (2019). Effect of gemcitabine and nab-Paclitaxel with or without hydroxychloroquine on patients with advanced pancreatic cancer: a phase 2 randomized clinical trial. JAMA Oncology, 5(7), 993–998. https://doi.org/10.1001/jamaoncol.2019.0684.
Diop-Frimpong, B., Chauhan, V. P., Krane, S., Boucher, Y., & Jain, R. K. (2011). Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proceedings of the National Academy of Sciences of the United States of America, 108(7), 2909–2914. https://doi.org/10.1073/pnas.1018892108.
Cun, X., Ruan, S., Chen, J., Zhang, L., Li, J., He, Q., & Gao, H. (2016). A dual strategy to improve the penetration and treatment of breast cancer by combining shrinking nanoparticles with collagen depletion by losartan. Acta Biomaterialia, 31, 186–196. https://doi.org/10.1016/j.actbio.2015.12.002.
Murphy, J. E., Wo, J. Y., Ryan, D. P., Clark, J. W., Jiang, W., Yeap, B. Y., et al. (2019). Total neoadjuvant therapy with FOLFIRINOX in combination with losartan followed by chemoradiotherapy for locally advanced pancreatic cancer: a phase 2 clinical trial. JAMA Oncology, 5(7), 1020–1027. https://doi.org/10.1001/jamaoncol.2019.0892.
Lee, K., Molenaar, R. J., Klaassen, R., Bijlsma, M. J., Weterman, M. J., Richel, D. J., et al. (2017). A Phase I study of LDE225 in combination with gemcitabine and nab-paclitaxel in patients with metastasized pancreatic cancer. Annals of Oncology. https://doi.org/10.1093/annonc/mdx369.143.
Yamamura, S., Matsumura, N., Mandai, M., Huang, Z., Oura, T., Baba, T., et al. (2012). The activated transforming growth factor-beta signaling pathway in peritoneal metastases is a potential therapeutic target in ovarian cancer. International Journal of Cancer, 130(1), 20–28. https://doi.org/10.1002/ijc.25961.
Hau, P., Jachimczak, P., Schlingensiepen, R., Schulmeyer, F., Jauch, T., Steinbrecher, A., et al. (2007). Inhibition of TGF-beta2 with AP 12009 in recurrent malignant gliomas: from preclinical to phase I/II studies. Oligonucleotides, 17(2), 201–212. https://doi.org/10.1089/oli.2006.0053.
Hwang, L., Ng, K., Wang, W., & Trieu, V. N. (n.d.). An anti-TGF-beta-2 antisense primed tumors to subsequent chemotherapies. Journal of Clinical Oncology. 185. https://doi.org/10.1200/JCO.2016.34.15_suppl.e15727
Hoffman, A., Qadri, B., Frant, J., Katz, Y., Bhusare, S. R., Breuer, E., et al. (2008). Carbamoylphosphonate matrix metalloproteinase inhibitors 6: cis-2-aminocyclohexylcarbamoylphosphonic acid, a novel orally active antimetastatic matrix metalloproteinase-2 selective inhibitor—synthesis and pharmacodynamic and pharmacokinetic analysis. Journal of Medicinal Chemistry, 51(5), 1406–1414. https://doi.org/10.1021/jm701087n.
Dufour, A., Sampson, N. S., Li, J., Kuscu, C., Rizzo, R. C., Deleon, J. L., et al. (2011). Small-molecule anticancer compounds selectively target the hemopexin domain of matrix metalloproteinase-9. Cancer Research, 71(14), 4977–4988. https://doi.org/10.1158/0008-5472.CAN-10-4552.
Liang, H., Li, X., Chen, B., Wang, B., Zhao, Y., Zhuang, Y., et al. (2015). A collagen-binding EGFR single-chain Fv antibody fragment for the targeted cancer therapy. Journal of Controlled Release: Official Journal of the Controlled Release Society, 209, 101–109. https://doi.org/10.1016/j.jconrel.2015.04.029.
Ishihara, J., Ishihara, A., Sasaki, K., Lee, S. S.-Y., Williford, J.-M., Yasui, M., et al. (2019). Targeted antibody and cytokine cancer immunotherapies through collagen affinity. Science Translational Medicine, 11(487). https://doi.org/10.1126/scitranslmed.aau3259.
Brennen, W. N., Rosen, D. M., Wang, H., Isaacs, J. T., & Denmeade, S. R. (2012). Targeting carcinoma-associated fibroblasts within the tumor stroma with a fibroblast activation protein-activated prodrug. Journal of the National Cancer Institute, 104(17), 1320–1334. https://doi.org/10.1093/jnci/djs336.
Sun, Z., Li, R., Sun, J., Peng, Y., **ao, L., Zhang, X., et al. (2017). Matrix metalloproteinase cleavable nanoparticles for tumor microenvironment and tumor cell dual-targeting drug delivery. ACS Applied Materials & Interfaces, 9(46), 40614–40627. https://doi.org/10.1021/acsami.7b11614.
Egeblad, M., Nakasone, E. S., & Werb, Z. (2010). Tumors as organs: complex tissues that interface with the entire organism. Developmental Cell, 18(6), 884–901. https://doi.org/10.1016/j.devcel.2010.05.012.
Wang, H., Mislati, R., Ahmed, R., Vincent, P., Nwabunwanne, S. F., Gunn, J. R., et al. (2019). Elastography can map the local inverse relationship between shear modulus and drug delivery within the pancreatic ductal adenocarcinoma microenvironment. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 25(7), 2136–2143. https://doi.org/10.1158/1078-0432.CCR-18-2684.
Li, X., Shepard, H. M., Cowell, J. A., Zhao, C., Osgood, R. J., Rosengren, S., et al. (2018). Parallel accumulation of tumor hyaluronan, collagen, and other drivers of tumor progression. Clinical Cancer Research, 24(19), 4798–4807. https://doi.org/10.1158/1078-0432.CCR-17-3284.
Gonçalves-Ribeiro, S., Sanz-Pamplona, R., Vidal, A., Sanjuan, X., Guillen Díaz-Maroto, N., Soriano, A., et al. (2017). Prediction of pathological response to neoadjuvant treatment in rectal cancer with a two-protein immunohistochemical score derived from stromal gene-profiling. Annals of Oncology: Official Journal of the European Society for Medical Oncology, 28(9), 2160–2168. https://doi.org/10.1093/annonc/mdx293.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Martins Cavaco, A.C., Dâmaso, S., Casimiro, S. et al. Collagen biology making inroads into prognosis and treatment of cancer progression and metastasis. Cancer Metastasis Rev 39, 603–623 (2020). https://doi.org/10.1007/s10555-020-09888-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-020-09888-5